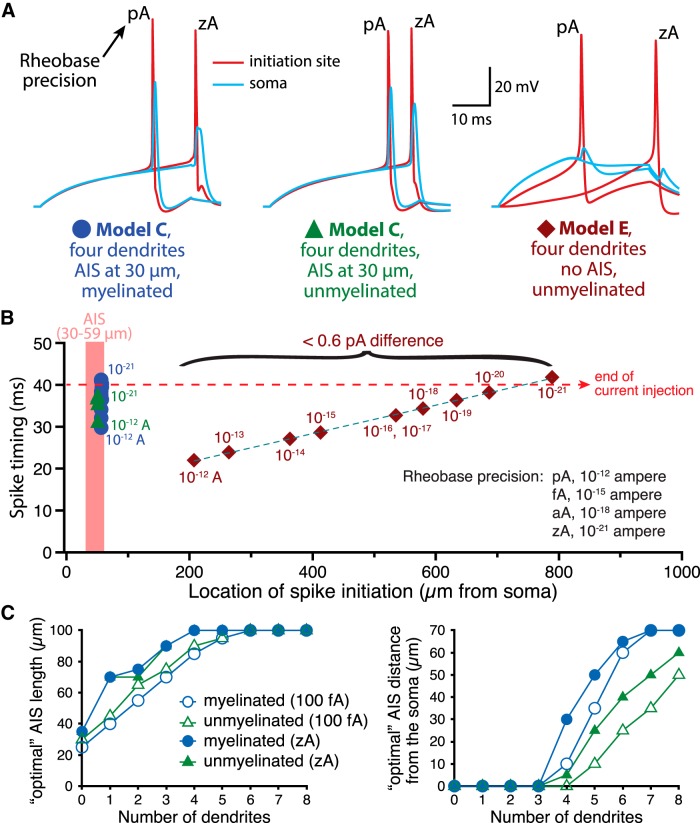
Figure 14.
Impact of rheobase precision on action potential initiation and AIS plasticity. A, Plots of action potential waveforms recorded in the AIS (red; 30 µm long and 30 µm from the soma) or in the soma (blue) of neurons with four dendrites and either an AIS associated with a myelinated axon (left), an unmyelinated (middle) axon (AIS Model C), or an unmyelinated axon lacking an AIS (AIS Model E; right). Shown are traces in which rheobase current was calculated to the nearest pA or to the nearest zA, as indicated (simulations used 100 ns time-steps). B, Plots of spike latency vs the location of spike initiation in myelinated and unmyelinated neurons having an AIS (Model C), or in a neuron lacking an AIS (Model E), for rheobase current injections at the indicated precision levels (i.e, rounded up to the nearest suprathreshold stated unit). Note that approaching ever more precise rheobase currents increased the latency to spike initiation and, in the absence of an AIS, moved the location of initiation to very distal regions of the axon. C, Plots of AIS lengths (left) or locations (right) having the lowest rheobase currents, as determined to the nearest 100 fA (open symbols) or 1 zA (closed symbols) in myelinated and unmyelinated neurons.