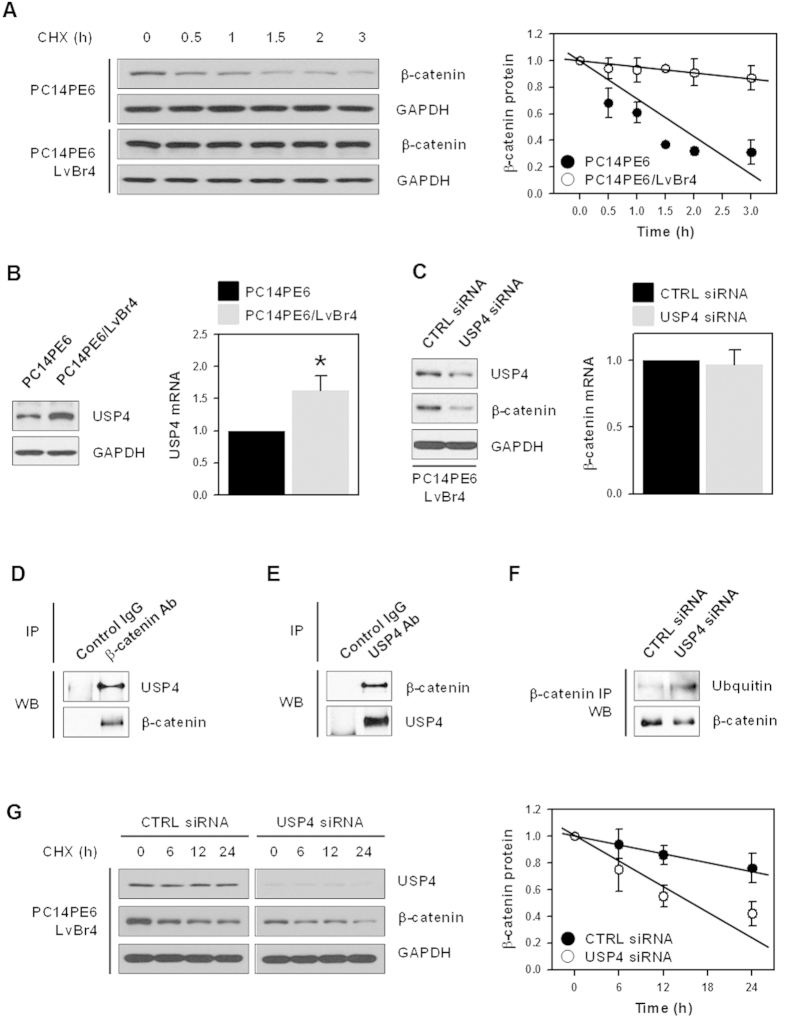
Figure 3. USP4 regulated the expression of β-catenin by controlling its protein stability.
(A) To compare the β-catenin protein stability, PC14PE6 and PC14PE6/LvBr4 cells were treated with cycloheximide (20 μg/mL) and harvested at the indicated times. Whole cell lysates were prepared, and the level of β-catenin protein was determined by western blotting. The stability of β-catenin was assessed by image analysis. (B) The expression level of USP4 in PC14PE6 and PC14PE6/LvBr4 cells was examined by western blotting (left panel) and RT-qPCR (right panel). (C) To determine whether USP regulates the expression of β-catenin, brain metastatic PC14PE6/LvBr4 cells were transfected with control (CTRL) or USP4-specific siRNA for 48 h. The whole cell extract was prepared and the expression level of USP4 and β-catenin was determined by western blotting. (D,E) To evaluate the direct interaction between USP4 and β-catenin, whole cell lysates were prepared using RIPA buffer, and equal amounts of protein were incubated with appropriate control IgG or an antibody against USP4 (D) or β-catenin (E). The level of β-catenin and USP4 in IP materials was assessed by western blotting. (F) To check the effect of USP4 silencing on the ubiquitination of β-catenin, PC14PE6 cells were transfected with control (CTRL) or USP4-specific siRNA for 48 h. Whole cell lysates were prepared using RIPA buffer and incubated with appropriate control IgG and β-catenin antibody. The levels of β-catenin and ubiquitin were assessed by western blotting. (G) To compare the protein stability of β-catenin, parental PC14PE6 and brain metastatic PC14PE6/LvBr4 cells were treated with cycloheximide (40 μg/mL) and harvested at the indicated times. The stability of β-catenin was determined as described above. Data are means and standard deviation from more than three independent experiments. *p < 0.05.