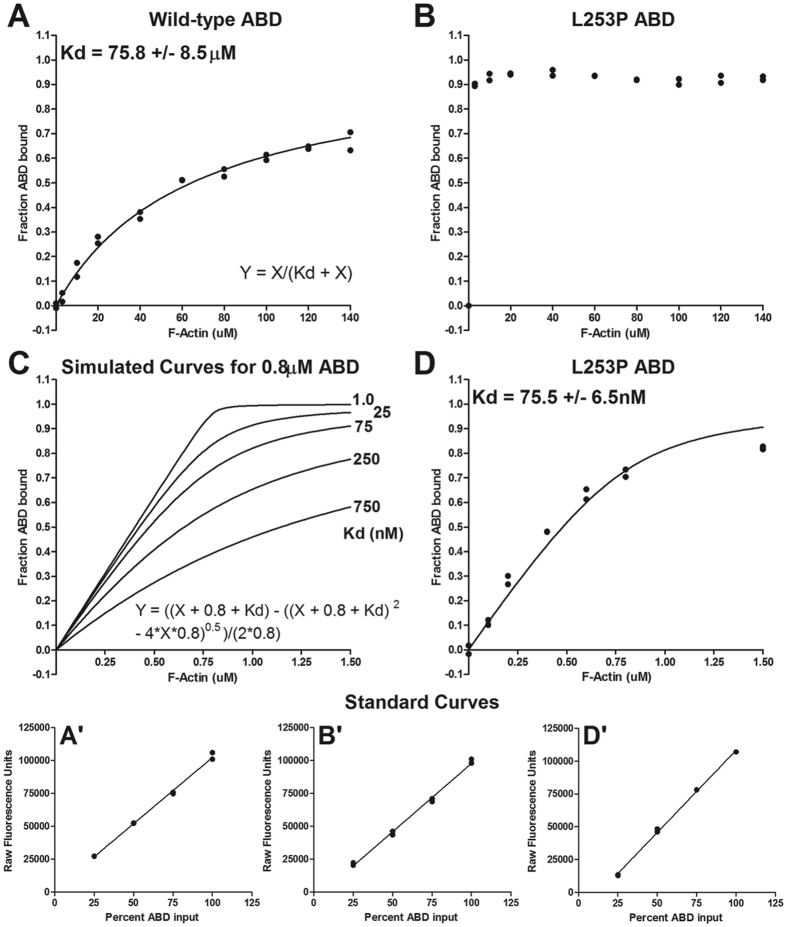
Figure 3. The L253P ABD binds F-actin with high affinity.
(A) Results of an individual co-sedimentation assay performed using 2 μM wild-type β-III-spectrin ABD. The wild-type ABD has a Kd of 75.8 μM (average +/− standard deviation). This is the average Kd from six co-sedimentation assays using two ABD protein preps and four different F-actin preps. Curve fitting was performed using the equation given in this panel. (B) Results of an individual co-sedimentation assay performed using 2 μM L253P ABD and same range of F-actin concentrations as used for wild-type. Binding of the L253P ABD under these conditions was tested in three co-sedimentation assays with similar results. (C) Simulated curves using the alternative equation given in this panel showed that a sub-micromolar Kd estimate for the L253P ABD could be attained using 0.8 μM L253P ABD protein and low concentrations of F-actin. (D) Results of an individual co-sedimentation assay showing the binding of 0.8 μM L253P ABD to low concentrations of phalloidin stabilized F-actin. Data were fit to the equation given in panel (C) The Kd is the average Kd from three co-sedimentation assays using L253P ABD purified for a second time following the initial binding assays in panel B. A’,B’ and D’ are standard curves used to convert Coomassie blue fluorescence intensities to amount wild-type or L253P ABD protein for the binding data shown in panels (A,B,D) respectively.