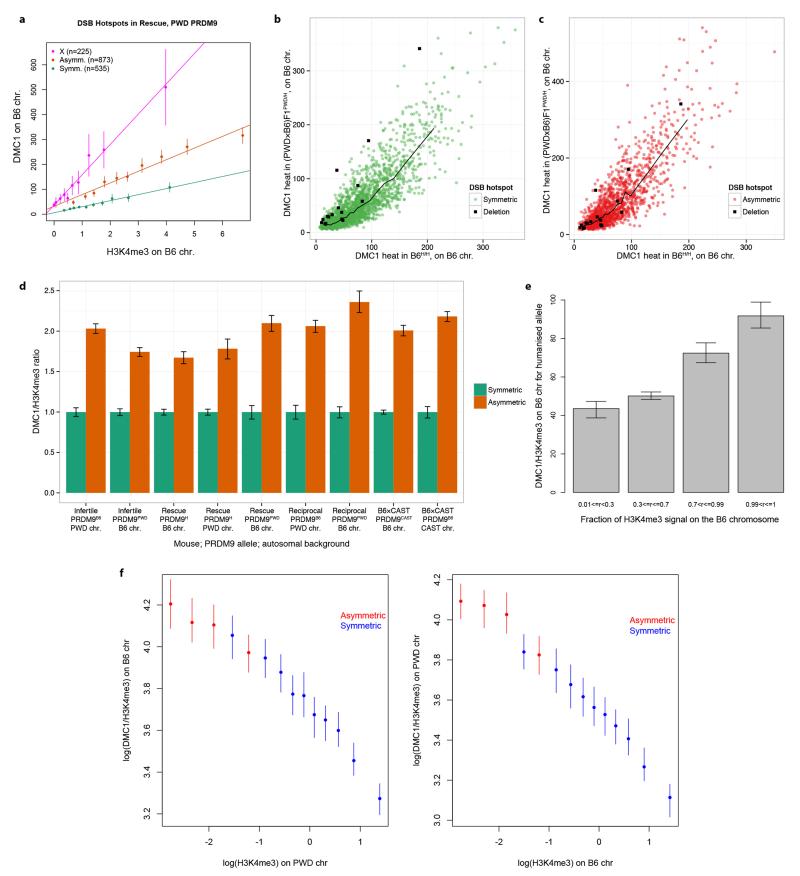
Extended Data Figure 9. Asymmetric hotspots, hotspots on the X chromosome and hotspots opposite deletions show systematic increase of DMC1 heat, relative to symmetric hotspots.
a, For the PWD allele in the humanized rescue (PWD×B6)F1PWD/H mouse, mean DMC1 signal is plotted in decile bins of H3K4me3 enrichment on the B6 chromosome (or the PWD X-chromosome), with error bars showing 95% CIs and lines of best fit (as in Fig. 4c). The slope of the line for asymmetric hotspots is 2.5-fold greater than that of the symmetric hotspots, and the slope for hotspots on the X-chromosome is 5.2-fold greater, illustrating that the DMC1 signal at asymmetric sites is elevated in a similar fashion to hotspots on the X-chromosome, which do not repair until late in meiosis. We found similar results in all cases tested. b, Comparison of DMC1 heats on B6 chromosome for hotspots shared between the humanized B6H/H and the humanized rescue (PWD×B6)F1PWD/H mice, under humanized PRDM9 control. We show symmetric hotspots (fraction of DMC1 informative reads between 0.4 and 0.6, green), and hotspots opposite a deletion on the PWD chromosome (deletion of at least 200bp, encompassing a human PRDM9 binding motif, black). The black line represents the median DMC1 heat for symmetric hotspots. c, As b, but showing the asymmetric hotspots (fraction of DMC1 informative reads above 0.9, red), with the corresponding median line. Hotspots opposite PWD deletion show a significant elevation in DMC1 heat relative to symmetric hotspots (14/16 hotspots above the symmetric median line, p=0.004). This elevation is similar to the one showed by asymmetric hotspots (9/16 hotspots above the asymmetric median line, p=0.80). d, Barplot showing the genome-wide ratio of mean DMC1 heat to mean H3K4me3 enrichment for asymmetric hotspots relative to symmetric hotspots in 9 scenarios studied, each for a different combination of mouse, Prdm9 allele, and haplotype, with error bars representing 95% bootstrap CIs for the ratio of means. In all cases, asymmetric hotspots show an elevation in DMC1 signal for a given H3K4me3 signal. e, Ratio of mean DMC1 and H3K4me3 signals on the B6 chromosome for the humanized allele in the humanized rescue mouse. Hotspots are clustered according to the fractions of their H3K4me3 signal that is on the B6 chromosome (r), and the ratio of the mean DMC1 and H3K4me3 signals in each class is shown here. The whiskers show 95% CIs for the mean, estimated using bootstrapping. When r>0.5, the B6 chromosome has greater H3K4me3 than the PWD chromosome, and vice versa. The ratio could not be estimated for r<=0.01 due to H3K4me3 levels being zero or nearly zero in those cases. f, (Left) Ratio of mean DMC1 and H3K4me3 signals on the B6 chromosome compared with the H3K4me3 signal on the PWD chromosome (log scale) in the infertile mouse. Asymmetric hotspots were defined as those with H3K4me3 fraction on the B6 chromosome > 0.9, and symmetric hotspots were those with the fraction between 0.1 and 0.9. Hotspots that we estimated to be completely asymmetric (H3K4me3 fraction=0 on either chromosome) or those with H3K4me3 enrichment on either chromosome close to zero (enrichment < 0.05) were excluded to avoid singularities on either axis. Asymmetric hotspots were binned into 4 bins of equal size and symmetric hotspots were binned into 10 bins of equal size. Different numbers of bins were used for asymmetric and symmetric hotspots to get approximately similar confidence intervals (error bars represent 95% CIs) to enable comparison. We did not observe many weak symmetric hotspots as we have limited power to detect such hotspots, which is why there are no symmetric bins with very low H3K4me3 levels on the homologue (Right). As (Left), but with the ratio determined for the PWD chromosome relative to H3K4me3 on the B6 chromosome. Accordingly, asymmetric hotspots are defined as those with H3K4me3 fraction on the PWD chromosome > 0.9.