Table 2. Pronucleophile and ligand screening in the isocyanoacetate aldol reaction between 8 and 7 a .
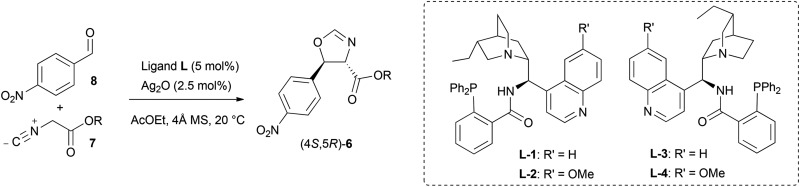
| |||||||
| Entry | R | 7 | Ligand | Time (min) | Yield b (%) | d.r. c (trans : cis) | e.e. d (%) |
| 1 | (CH3)3C | 7a | L-1 | 30 | 70 | 91 : 9 | 78 |
| 2 | CH3 | 7b | L-1 | 100 | 80 | 88 : 12 | 82 |
| 3 | PhCH2 | 7c | L-1 | 180 | 61 | 90 : 10 | 87 |
| 4 | PhCH2 | 7c | L-2 | 80 | 64 | 90 : 10 | 87 |
| 5 | 4-(OCH3) C6H4CH2 | 7d | L-2 | 60 | 63 | 89 : 11 | 86 |
| 6 | 3,5-(CF3)2 C6H3CH2 | 7e | L-2 | 60 | 56 | 90 : 10 | 84 |
| 7 | Ph2CH | 7f | L-1 | 100 | 81 | 93 : 7 | 87 |
| 8 | Ph2CH | 7f | L-2 | 45 | 78 | 91 : 9 | 89 |
| 9 | Ph2CH | 7f | L-3 | 200 | 82 | 93 : 7 | 88 e |
| 10 | Ph2CH | 7f | L-4 | 60 | 68 | 92 : 8 | 93 e |
aReaction performed on 0.25 mmol of 7 (0.01 M in AcOEt) using 1.1 eq. of 8. Configuration of 6 assigned by analogy with previous work.9
bIsolated yield of trans diastereomer after FCC.
cd.r. determined by 1H NMR analysis of the crude reaction mixture.
de.e. of trans diastereomer determined by HPLC on chiral stationary phase.
eOpposite enantiomer obtained.
