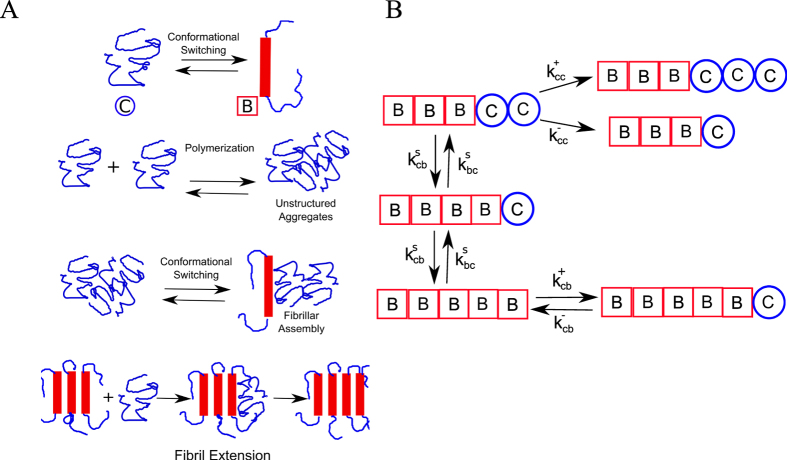
Figure 1.
(A) A schematic depiction the phenomenon of amyloid-like self assembly by proteins/peptides. Initially, free monomers in solution are in their soluble form (state ‘C’). These monomers can stochastically self-associate to give rise to unstructured aggregates. Upon aggregating, they can undergo structural transition from a coil like state ‘C’ to a β-sheet like form (state ‘B’). These insoluble β-sheet rich assemblies are more stable due to specific H-bonded interactions and continue to grow by recruiting more monomers from solution. The initial state of the peptides in solution is its soluble form ‘C’. (B) The two-state model for amyloid formation showing the various processes implemented in this model. A polymerization event wherein a random-coil monomer ‘C’ binds to a random coil monomer at the fibril edge 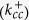 , the switching of a C monomer to a B monomer on the filament
, the switching of a C monomer to a B monomer on the filament 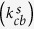 , the switching of a B monomer to a C monomer on the filament
, the switching of a B monomer to a C monomer on the filament 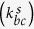 and the binding of a free C monomer to a B monomer on the fibril edge
and the binding of a free C monomer to a B monomer on the fibril edge 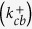 are shown in the figure. The depolymerization of a ‘C’ monomer bound to a ‘C’ monomer and a ‘C’ monomer bound to a ‘B’ monomer are
are shown in the figure. The depolymerization of a ‘C’ monomer bound to a ‘C’ monomer and a ‘C’ monomer bound to a ‘B’ monomer are 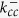 and
and 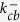 , respectively. ‘C’ and ‘B’ refer to the coil-like and β-strand conformations of the peptide, respectively.
, respectively. ‘C’ and ‘B’ refer to the coil-like and β-strand conformations of the peptide, respectively.