Fig 1.
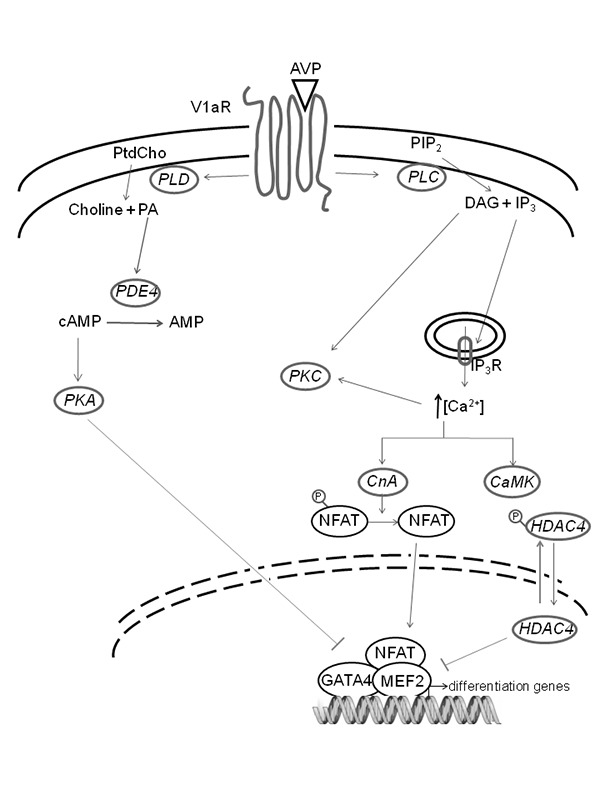
Schematic representation of AVP-dependent signaling pathways in myogenic cells. The transduction of AVP signal in myogenic cells involves the activation of PLC and PLD. PLC hydrolyzes phosphatidylinositol 4,5-bisphosphate, producing the second messengers DAG and IP3. DAG also derives from PA produced by PtdCho via PLD. DAG activates the protein kinase C (PKC), while IP3 is responsible for Ca2+ release from the endoplasmic reticulum into the cytosol. An increased intracellular cytosolic Ca2+ concentration activates the CaMK pathway, thus inducing cytosolic compartmentalization of class II HDACs. Calcium signaling also activates CnA, which in turn promotes the dephosphorylation and the consequent nuclear import of the NFAT family. In the nucleus, NFAT gives rise to the formation of multifactorial complexes with MEF2 and GATA2 in the promoter region of muscle specific genes. The absence of class II HDACs is also likely to promote this transcriptional activation, leading to the activation of the skeletal muscle differentiation program. AVP also interferes with the cAMP signaling system: PA (produced by PLD-dependent hydrolysis of PtdCho) activates PDE4, which in turn promotes cAMP breakdown, thus inhibiting cAMP-dependent activation of PKA, a kinase known to negatively regulate myogenic differentiation.
