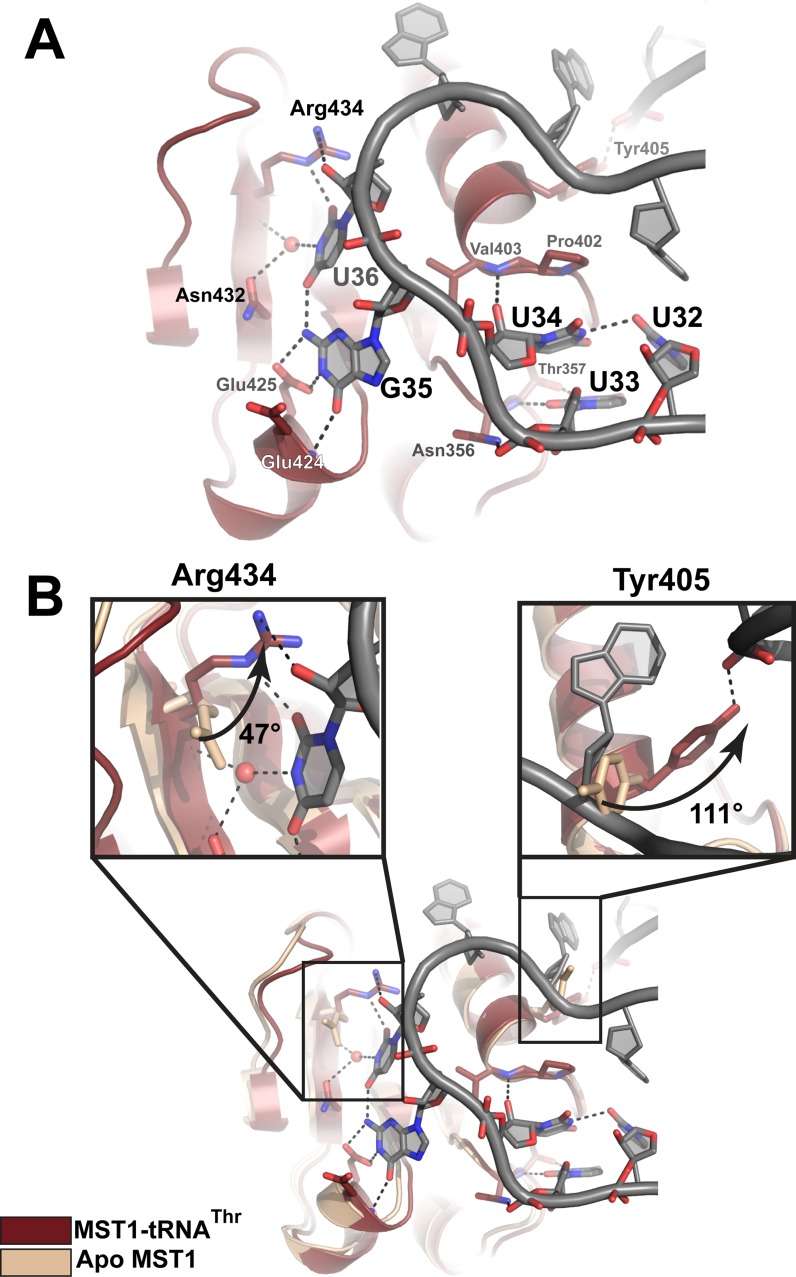
Figure 4.
Recognition of the anticodon sequence and structural rearrangements in the anticodon-binding domain of MST1 upon complex formation. (A) The first position in the anticodon sequence, U34, is not specifically recognized by MST1. Instead, Thr357 recognizes U33, the base upstream of the anticodon. The second base, G35, is recognized by the backbone amide of Glu424 and Glu425, whereas the third position, U36, is recognized by Asn435, the backbone atoms of Arg434, and a water molecule. The backbone atoms of MST1 and tRNA are red and grey, respectively. (B) Superimpositioning of apo MST1 (PDB ID 3UGQ; beige) onto the MST1-tRNA complex (red and grey) reveals structural rearrangements in the anticodon-binding domain upon tRNA binding. The first inset highlights the movement of Arg434 that is important for recognition of U36. The side chain of Arg434 rotates ∼47° and translates ∼1.4 Å toward the tRNA. The second inset shows rotation (∼111°) of Tyr405. This movement positions the side-chain hydroxyl within the H-bonding distance from the sugar-phosphate backbone of A26 in the anticodon stem.