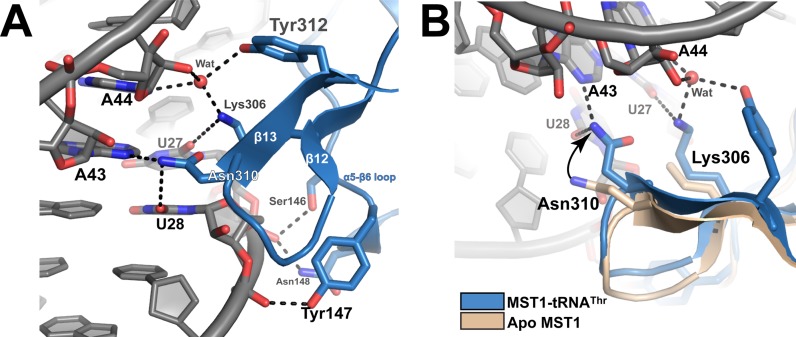
Figure 5.
Cross dimer interactions between MST1-B and the anticodon stem of tRNA-A stabilize the ternary complex. (A) The catalytic domain of MST1-B establishes sequence-specific contacts with the anticodon stem of tRNA-A. The side chains of Lys306 and Tyr312 participate in recognition of A44. Lys306 is also involved in ‘reading’ the identity of U27, whereas Asn310 makes base specific contacts with U28 and A43. (B) The structural overlay of apo (PDB ID: 3UGQ; beige) and tRNA-bound MST1 (blue and grey) reveals that residues of the catalytic domain of MST1-B that ‘read’ the sequence of the anticodon stem undergo a conformational change upon ternary complex formation. Asn310 moves 3.3 Å from its position in the apo structure to interact with U28 and A43. Lys306 is partially disordered in apo MST1, but it is ordered in the ternary complex in which it interacts with a water molecule and the base of U27.