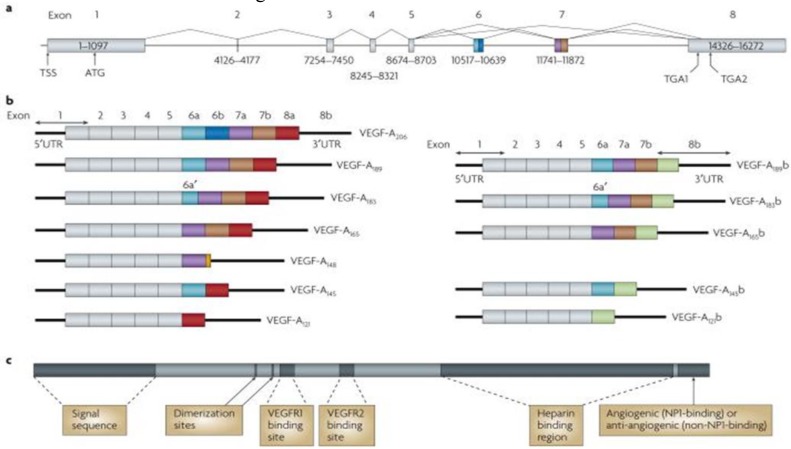
Fig. 1.
Protein and mRNA products of human vascular endothelial growth factor A (VEGF-A)(11). a Gene structure of human VEGF-A. VEGF-A spans 16,272 bp of chromosome 6p21.1 and consists of eight exons. Alternate 5′ and 3′ splice site selection in exons 6, 7, and 8 generate multiple isoforms. Exons 6 and 7 encode heparin-binding domains. The transcriptional start site (TSS) and translational start site (ATG) in exon 1 are indicated. Alternative stop codons within exon 8 are also indicated (TGA1 and TGA2). b | Alternative splicing can occur either at the 5′ donor splice site (for example, VEGF-A189 versus VEGF-A206) or the 3′ acceptor splice site (for example, VEGF-A189 versus VEGF-A165). Two mRNA isoform families are generated. The pro-angiogenic isoforms (VEGF-Axxx, left) are generated by proximal splice site (PSS) selection in exon 8 and the anti-angiogenic family (VEGF-Axxxb, right) from exon 8 distal splice site (DSS) choice. Thus, VEGF-A165, formed by PSS selection in exon 8, has VEGF-A165b as its DSS sister isoform, the DSS-selected mRNA encoding a protein of exactly the same length. Exon 6a’ occurs in VEGF-A183 as a result of a conserved alternative splicing donor site in exon 6a and is 18 bp shorter than full-length exon 6a. VEGF-A148 is a truncated isoform splicing from exon 7a into exon 8a out of frame and resulting in a premature stop codon. VEGF-A206b has not yet been identified. c | Protein structure of VEGF-A containing the dimerization sites and binding sites for heparin, VEGF-A receptor 1 (VEGFR1; encoded by exon 3) and VEGFR2 (encoded by exon 4), which are present in all isoforms. The six amino acids at the extreme carboxyl terminus of the protein can be either pro-angiogenic (CDKPRR, encoded by exon 8a) or anti-angiogenic (SLTRKD, encoded by exon 8b). The epitopes recognized by most commercial antibodies are in the region of the VEGF-A receptor-binding domains, present in VEGF-A isoforms of both families. UTR, untranslated region [ Adapted from (11)].