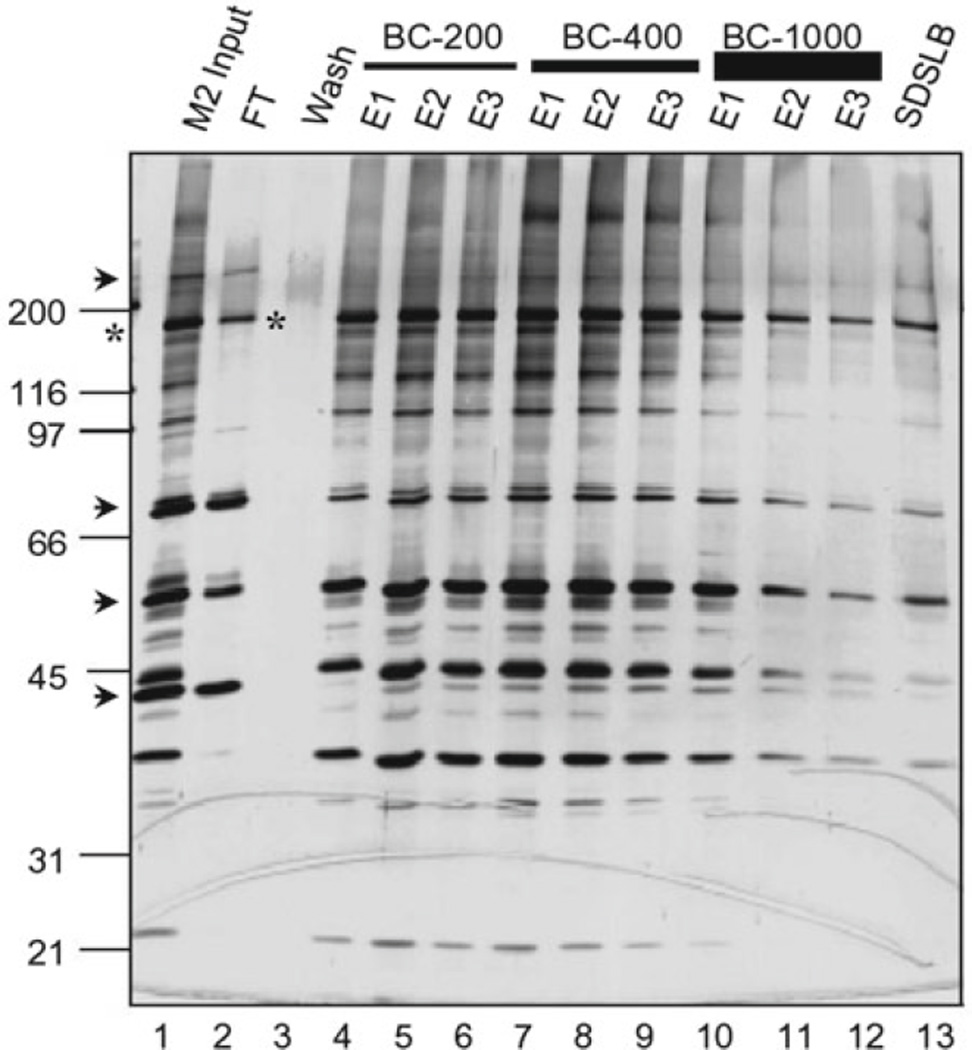
Fig. 1.
S-Sepharose fractionation of anti-FLAG/M2-purified ATAC complexes. Shown is a silver-stained, SDS-PAGE gel containing different S-Sepharose elution fractions. M2-purified, FLAG-tagged ATAC complexes (M2 input, lane 1) were adsorbed onto S-Sepharose resin in BC-60, washed with BC-100 (Wash, lane 3), and eluted successively with three resin volumes (E1–E3) of the indicated BC buffers containing from 200 mM NaCl (BC–200) to 1,000 mM NaCl (BC-1000) and finally with SDS loading buffer (SDSLB). FT unbound “flow through” fraction. Proteins that bind nonspecifically to M2 agarose (arrow-heads) and contaminate the M2-purified complexes (M2 input) do not bind to S-Sepharose (i.e., present in FT fraction, lane 2). Excess, “free,” FLAG-tagged YEATS2 subunit (asterisks) does not bind to S-Sepharose either (lane 2).