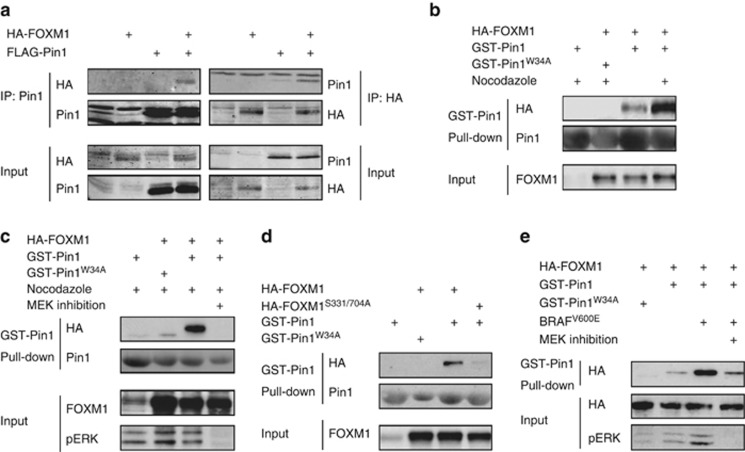
Figure 4.
Pin1 physically regulates FOXM1 in a MEK-dependent manner. (a) Pin1 and FOXM1 physically interact. FLAG-Pin1 and HA-FOXM1 were transiently expressed in U2OS cells (input) and subjected to immunoprecipitation (IP) with anti-HA or anti-FLAG antibodies. Immunoblot analysis was performed with antibodies against Pin1 or HA to detect FOXM1. (b) The Pin1-FOXM1 interaction increases at the G2/M boundary of the cell cycle. U2OS cells transiently expressing HA-FOXM1 were treated for 24h with 250ng/ml Nocodazole to synchronize them at G2/M and lysates were subjected to a pull-down using recombinant GST-Pin1 or the substrate binding-deficient GST-Pin1W34A. Immunoblot analysis was performed as in a. (c) The Pin1-FOXM1 interaction is MEK-dependent. U2OS cells were treated as in e, but in the presence or absence of a 24 h pretreatment with a MEK inhibitor (10 μM U0126). (d) The MEK-target sites, S331 and S704, are essential for the Pin1-FOXM1 interaction. U2OS cells expressing wild-type HA-FOXM1 or a mutant in which Ser331 and Ser704 are mutated to Ala (HA-FOXM1S331/704A) were processed as described in (b). (e) BRAFV600E stimulates the Pin1-FOXM1 interaction through MEK-dependent modification of FOXM1. U2OS cells expressing HA-FOXM1 and BRAFV600E were left untreated or treated with for 24 h with a MEK inhibitor (20 μM PD184352) and processed as in (b).