Abstract
Background
Exercise is recommended for people with diabetes, but little is known about exercise in people with diabetic peripheral neuropathy (DPN).
Objective
The primary purpose of this preliminary study was to examine adverse events (AEs) during moderate-intensity, supervised aerobic exercise in people with DPN. The secondary purpose was to examine changes in fatigue, aerobic fitness, and other outcomes after intervention.
Design
This was a single-group preliminary study.
Setting
The setting was an academic medical center.
Participants
Participants were 18 people who were sedentary and had type 2 diabetes and peripheral neuropathy (mean age=58.1 years, SD=5).
Intervention
The intervention was a supervised 16-week aerobic exercise program (3 times per week at 50% to >70% oxygen uptake reserve).
Measurements
Adverse events were categorized as related or unrelated to the study, anticipated or unanticipated, and serious or not serious. Outcomes included fatigue (Multidimensional Fatigue Inventory), cardiovascular fitness (peak oxygen uptake), body composition (dual-energy x-ray absorptiometry), sleep quality, plasma metabolic markers, and peripheral vascular function.
Results
During the study, 57 nonserious AEs occurred. Improvements were found in general fatigue (mean change=−3.5; 95% confidence interval [95% CI]=−1.3, −5.3), physical fatigue (mean change=−3.1; 95% CI=−1.2, −5.0), peak oxygen uptake (mean change=1.1 mL·kg−1·min−1; 95% CI=0.2, 1.9), total body fat (mean change=−1%; 95% CI=−0.3, −1.7), fat mass (mean change=−1,780 g; 95% CI=−616.2, −2,938.7), and peripheral blood flow (mean change=2.27%; 95% CI=0.6, 4.0).
Limitations
This was a small-scale, uncontrolled study. A future randomized controlled trial is needed to fully assess the effects of exercise on the outcomes.
Conclusions
This study provides new support for supervised aerobic exercise in people with DPN. However, it is important for physical therapists to carefully prescribe initial exercise intensity and provide close monitoring and education to address the anticipated AEs as people who are sedentary and have DPN begin an exercise program.
Diabetic peripheral neuropathy (DPN) is one of the most common complications of diabetes, with lower limb pain and sensory loss caused by slow peripheral nerve degeneration. The most common form of DPN is symmetrical distal degeneration of peripheral nerves combined with impaired nerve regeneration.1 As a consequence of DPN, people with long-standing diabetes may have pain or significant deficits in tactile sensitivity, vibration sense, and lower-limb joint proprioception. These sensory deficits may contribute to impaired balance and gait, with increased risk of falls, lower extremity injury, and amputation.2–5 People with DPN are more likely to be sedentary and to have decreased daily walking distances.6
Several large-scale randomized controlled trials have established that aerobic exercise improves physical fitness, glycemic control, and insulin sensitivity in people with diabetes.7–10 However, people with DPN may not respond to exercise in the same way. Neuropathic pain may interfere with a person's willingness to participate in an exercise program, and there is evidence that neuropathy also may interfere with pain modulation during exercise.11 As recently as 2008, the Standards of Medical Care in Diabetes position statement published by the American Diabetes Association stated that only non–weight-bearing activities should be encouraged in people with DPN.12 Current guidelines have been adjusted because moderate-intensity weight-bearing activities do not appear to increase the risk of foot ulcers in people with DPN.13,14 The physical activity target for adults with diabetes is a minimum of 150 minutes of moderate-intensity aerobic activity (ie, 50%–70% of the maximal heart rate [HR]) per week.15,16 However, no studies have identified the most appropriate rate of progression to achieve this level in people who are sedentary and have type 2 diabetes, as noted by Colberg et al.16
Few studies have examined whether exercise provides specific benefits for glycemic control in people with DPN,17,18 although emerging research has shown promising effects of exercise on cutaneous reinnervation and decreased neuropathic symptoms in small, uncontrolled trials.18,19 Several larger randomized controlled trials in older adults with DPN have demonstrated that exercise results in increased levels of activity in the home,14,17 with inconsistent results for balance and fall risk.20–23 No studies have examined the use of aerobic exercise to improve several other relevant outcomes in people with DPN, including fatigue, cardiovascular fitness, sleep quality, body fat, plasma lipids, and vascular function. Little is known about the need for physical therapist supervision or monitoring during exercise interventions for people with DPN.
Fatigue is a common symptom in people with diabetes, reported by 24.6% of adults with type 2 diabetes in a recent large-scale longitudinal survey.24 In addition to fatigue being more common, people with diabetes report higher levels of fatigue than people without diabetes,25 and older adults who had diabetes and reported fatigue had lower survival rates 24 months later than people without fatigue.24 A relationship between fatigue and cardiovascular fitness has been found in people with other chronic neurologic conditions, such as stroke.26 People with DPN may have increased fatigue because of avoidance of physical activity and decreased cardiovascular fitness as a result of the complicating effects of diabetes, obesity, lower limb pain, and sensory loss.
Because little is known about the complications that are related to pain, comorbidities, and diabetes and that may occur when people with DPN initiate an exercise program, the primary purpose of this preliminary study was to report the frequency, type, and severity of adverse events (AEs) during a moderate-intensity, 16-week supervised aerobic exercise intervention in people with DPN. The secondary purpose was to examine changes in fatigue and related outcome measures after the intervention. Although this was an exploratory study, we hypothesized that we would find improvements in self-reported fatigue (Multidimensional Fatigue Inventory [MFI-20]) and increases in aerobic fitness (peak oxygen uptake [V̇o2peak] during a graded exercise test) after the intervention.
Method
This nonrandomized, single-group preliminary study utilized a preintervention-postintervention measurement design, with all enrolled participants taking part in the intervention. The study was completed at the University of Kansas Medical Center, with data collection between June 2012 and July 2013.
Participants
Potential study participants were identified either by self-referral in response to study advertisements or through the Frontiers Research Participation Registry program (supported by a National Institutes of Health–funded Clinical and Translational Science Award). People who were 40 to 70 years of age, who reported a diagnosis of type 2 diabetes with symptoms of neuropathy, and who were interested in the study were screened by a phone interview. The phone interview included the Telephone Assessment of Physical Activity27 to identify participants who were sedentary or underactive, as determined by a score of ≤5. Those who qualified provided consent by signing an institutionally approved informed consent form and then underwent further screening before enrollment to confirm the absence of the following exclusionary criteria: serious cardiac pathology, unstable hypertension, or serious musculoskeletal problems that would limit the ability to exercise; skin conditions, circulatory insufficiency, or open wounds on the leg; inability to ambulate independently; stroke or other central nervous system pathology; body weight of greater than 202.5 kg (450 lb); inadequate cognition and communication abilities, defined as scores of less than 24 on the Mini-Mental State Examination; or pregnant or planning on becoming pregnant, confirmed with a urine pregnancy test for women who were premenopausal. Because a skin biopsy also was obtained for analysis of intraepidermal nerve fiber density and nerve growth factors (not reported here), additional exclusionary criteria included lidocaine allergy and blood clotting disorder.
After consent but before enrollment, a clinical examination was performed by a neurologist (M.P.) to confirm the presence of DPN on the basis of standard criteria28: “possible” (symptoms or signs of DPN), “probable” (symptoms and signs of DPN), or “confirmed” (abnormal nerve conduction with symptoms and signs of DPN). Participants also completed a graded maximal exercise test with integrated electrocardiography to confirm their ability to exercise safely before enrollment and to provide baseline V̇o2peak values (more details about this test are provided in the description of cardiovascular fitness outcomes). Once the participants were enrolled, their primary care physicians were notified of their involvement in the study.
Interventions
All participants were scheduled for 16 weeks of supervised aerobic exercise 3 times per week (Tab. 1). The intensity of the exercise session was individually prescribed on the basis of the HR response at the corresponding 50% to 70% of oxygen uptake (V̇o2) reserve from the graded exercise test. The target V̇o2 reserve was calculated from the test with the following formula: [(maximal V̇o2 − V̇o2 at rest) × % intensity] + V̇o2 at rest.29
Table 1.
Progression of Intensity and Target Heart Rate (HR) Duration During Aerobic Exercise Interventiona
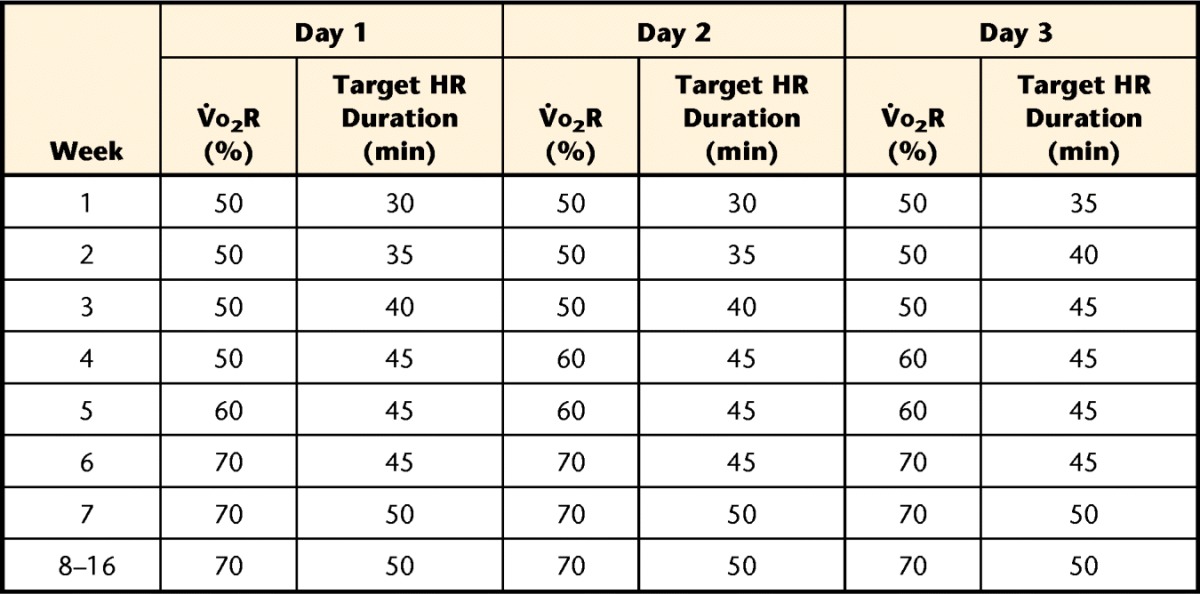
V̇o2R=oxygen uptake reserve, individually calculated for participants on the basis of the results of the graded maximal exercise test. Average heart rate target values were 102.4 (SD=11.7) beats per minute (bpm) for 50% V̇o2R, 109.5 (SD=13.3) bpm for 60% V̇o2R, and 115.8 (SD=14.7) bpm for 70% V̇o2R.
The use of V̇o2 reserve instead of V̇o2peak to calculate target intensity does result in a higher workload than the current guidelines of 50% to 70% of the maximal HR.15 However, we found that for most participants, the HR that corresponded to 50% of the maximal HR achieved during the exercise test was too close to the baseline to be an effective target during exercise. For a specific illustration, we provide data here for one participant. At rest, her HR was 64 bpm, and she achieved a maximal HR of 160 bpm at peak effort (19.6 mL·kg−1·min−1) during the exercise test. A target HR of 80 bpm would have been too close to the baseline to be effective for exercise; her HR was more than 80 bpm within the first 30 seconds of the exercise test. Therefore, we used the formula given above to calculate V̇o2 reserve at 50% for this participant to be: [(19.6 − 3.5 mL·kg−1·min−1) × 0.5] + 3.5 mL·kg−1·min−1=11.55 mL·kg−1·min−1. The corresponding HR for this participant at this workload was 105 bpm, which was used as her initial target for exercise.
Participants had access to a variety of aerobic training equipment, including cycle ergometers, treadmills, recumbent steppers, and elliptical trainers. Each session began with a 5- to 10-minute warm-up, ended with a 5- to 10-minute cool-down, and included the target HR duration noted in Table 1.
Sessions were supervised by licensed health care professionals or health care professional students, with no more than 4 participants per supervisor. Blood glucose level, blood pressure, HR, and rate of perceived exertion (RPE) were monitored during each session. A visual foot examination was performed once each week to ensure the absence of developing foot ulcers, and participants were encouraged to inspect their feet on a daily basis. If individual participants had difficulty achieving the target HR during the session, a moderate RPE level (11–13 on the 17-point scale)30 was used to indicate a comparable work level.31 Participants were not permitted to exercise if resting blood pressure was greater than 200 mm Hg (systolic) or greater than 110 mm Hg (diastolic). Responses to blood glucose levels followed recommended guidelines15:
If hypoglycemic (<100 mg/dL), carbohydrate snacks were provided, and glucose testing was repeated.
If hyperglycemic (>300 mg/dL) and the participant was not taking insulin, exercise was permitted with close monitoring of blood glucose every 10 to 15 minutes to ensure that the blood glucose level did not increase with activity.
If hyperglycemic (>300 mg/dL) and the participant was taking insulin, urine was checked for ketosis. If the ketone test result was positive, then exercise was postponed. If the ketone test result was negative, then exercise was permitted with close monitoring of blood glucose every 10 to 15 minutes.
Documentation of AEs
During each study visit, participants were asked about AEs, including falls, changes in medical status, or problems that occurred during study participation. These events were classified as either related or unrelated to study procedures; anticipated (included in consent form) or unanticipated (not included in consent form); and serious (grade 4 of Common Terminology Criteria for Adverse Events [CTCAE v3.0])32 or not serious (grade 1, 2, or 3 of CTCAE v3.0). A neurologist (M.M.D.) without financial interest in this project served as the independent data and safety monitor for this study. The number of participants screened or enrolled, the number of dropouts, and the number, frequency, and response to AEs were reviewed by the data and safety monitor quarterly during the project.
Outcomes
Assessments were completed at baseline and after the 16-week intervention.
Fatigue.
The Multidimensional Fatigue Inventory is a questionnaire consisting of 20 statements scored in 5 subscales: general fatigue, physical fatigue, mental fatigue, reduced motivation, and reduced activity.33 Reliability and validity were established in people with cancer and chronic fatigue syndrome,33,34 and this instrument was used in previous studies of fatigue in people with diabetes.35,36 Each item is scored from 1 to 5 (from agreement with the accompanying statement [“yes, that is true”] to disagreement [“no, that is not true”]). Total scores can range from the minimum of 4 to the maximum of 20, and a higher score indicates more fatigue.
Cardiovascular fitness.
Peak oxygen uptake during the graded exercise test was assessed with a metabolic cart (TrueOne 2400, Parvo Medics, Sandy, Utah) and integrated electrocardiography. A standardized exercise test protocol with a total body recumbent stepper (TBRS-XT) (T5XR, NuStep, Ann Arbor, Michigan)37 was administered by an exercise physiologist with a medical monitor present to monitor the test. Compared with a standard treadmill protocol, the TBRS-XT was validated and found to be reliable in adults who were healthy.37 The TBRS-XT consists of 2-minute stages, with gradually increasing workloads during the stages (eg, 50 W during stage 1, 75 W during stage 2, and 100 W during stage 3). Cadence was maintained at 115 steps per minute throughout the test.
In addition to continuous monitoring of HR and rhythm during the test, blood pressure and RPE were measured at the end of each stage (ie, 2-minute intervals) until maximal effort was achieved. Criteria for a “maximal” exercise test included reaching a plateau in V̇o2 with increased workload, a respiratory exchange ratio of greater than or equal to 1.15, a peak HR within 85% of the age-predicted maximal HR, and an RPE of greater than or equal to 18.29 The test was stopped before maximal effort was achieved if any of the following occurred: angina, dyspnea, fatigue (voluntary exhaustion or inability to maintain a stepping cadence of >110 steps per minute), hypertension (>250 mm Hg systolic or >115 mm Hg diastolic), hypotension, or ischemic electrocardiography abnormalities.
The same exercise physiologist administered tests both before and after the intervention and used standardized procedures to minimize bias (timed encouragement scripts, no review of preintervention test results before postintervention tests). The V̇o2peak (mL·kg−1· min−1) obtained from these tests was used as an outcome measure and to calculate a moderate intensity (50%–70% of V̇o2 reserve) for the aerobic training program as described previously.
Sleep quality.
The Pittsburgh Sleep Quality Index was used to measure sleep quality with a series of questions to arrive at a score ranging from 0 (very good) to 3 (very bad).38 This index was found to be a reliable and valid measure of sleep disturbances in a wide range of people.38–42
Body composition.
Participants were scanned with dual-energy x-ray absorptiometry (Lunar iDXA, GE Healthcare, a subsidiary of General Electric, Little Chalfont, Buckinghamshire, United Kingdom). Outcome measures included total body fat (percentage), fat mass (grams), lean mass (grams), visceral fat volume (cubic centimeters), visceral fat mass (grams), and bone mineral density (g/cm2).
Plasma insulin, glucose, and lipid levels.
Glycated hemoglobin A1c (HbA1c) was measured with a disposable fingerstick blood testing kit (A1CNow, Bayer, Whippany, New Jersey). After an overnight fast, blood was drawn for analysis of plasma insulin levels (mU/L), plasma glucose levels (mmol/L), and a standard lipid profile: total cholesterol, high-density lipoprotein, low-density lipoprotein, and triglycerides. The homeostasis model assessment– insulin resistance index was calculated from values for fasting plasma insulin (mU/L) and fasting plasma glucose (mmol/L) with the following equation: [fasting plasma insulin (mU/L) × fasting plasma glucose (mmol/L)]/22.5. Compared with the gold standard—the hyperinsulinemic-euglycemic clamp method—the homeostasis model assessment for insulin resistance was found to be a well-validated index of insulin resistance.43
Peripheral vascular function.
Flow-mediated dilation (FMD) in the brachial artery was assessed after an overnight fast with previously described methods.44 Participants were asked to refrain from food or caffeine for 12 hours and vigorous activity for 24 hours before the FMD procedure. Participants rested in the supine position for 20 minutes before the FMD procedure at a controlled temperature (22°C–24°C). An automated cuff with a rapid inflation system (DE Hokanson, Bellevue, Washington) was placed just distal to the olecranon process.45,46 Once a satisfactory image of the brachial artery was obtained, the transducer was stabilized with a custom-designed holder. Because we performed repeated scans, the location of the transducer was marked, and measurements from the olecranon process (reference point) were recorded to ensure identical location. Baseline diameter and blood flow velocity were recorded continuously for 60 seconds. Then, the automated cuff was inflated to 220 mm Hg, and the inflation was maintained for 5 minutes. Twenty seconds before cuff deflation, recordings of diameter and blood flow velocity were resumed. At 5 minutes, the cuff was deflated, and ultrasound images continued to be recorded for another 3 minutes. All images were stored on a computer and analyzed offline with specialized software (Brachial Analyzer, Medical Imaging Applications, Coralville, Iowa) and an automated mathematical algorithm to determine the peak FMD.
Data Management
Case report forms for this project were developed on the basis of general common data element forms developed for neuromuscular disease by the National Institutes of Health.47 Data from these forms were entered into a spreadsheet (Microsoft Excel 2010, Microsoft Corp, Redmond, Washington). A 100% quality audit was performed by the study team after the completion of data collection to ensure the accuracy of data entry.
Data Analysis
The frequency of AEs was determined for each participant and for all participants for each week of the intervention. No power analysis was completed for this study because it was an exploratory preliminary study. Data from the spreadsheet were imported into statistics software (IBM SPSS v20, IBM Corp, Armonk, New York) for analysis, and the alpha level was set at .05 for all statistical analyses. For each of the outcome measures, descriptive statistics (mean and standard deviation) were calculated, with identification of outliers. Normal distribution was confirmed through the Kolmogorov-Smirnov z statistic. Outcome measures obtained before the intervention were compared with those obtained after the intervention by use of a 2-tailed paired t test. For significant findings, the 95% confidence interval of the difference was also calculated. Additional analyses included comparison of participants who completed greater than 80% of the exercise sessions with those who completed less than 80% of the exercise sessions by use of an independent t test and determination of associations between exercise attendance and changes in outcomes by use of the Pearson (r) correlation coefficient.
Role of the Funding Source
This work was supported by Clinical and Translational Science Award (CTSA) program grants from the National Center for Advancing Translational Sciences (NCATS), awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research UL1TR000001 and TL1TR000120 (for M.Y.). Support also was provided by grants T32HD057850 (for L.J.D.) and K01HD067318 (for S.A.B.) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NCATS, or the National Institutes of Health.
Results
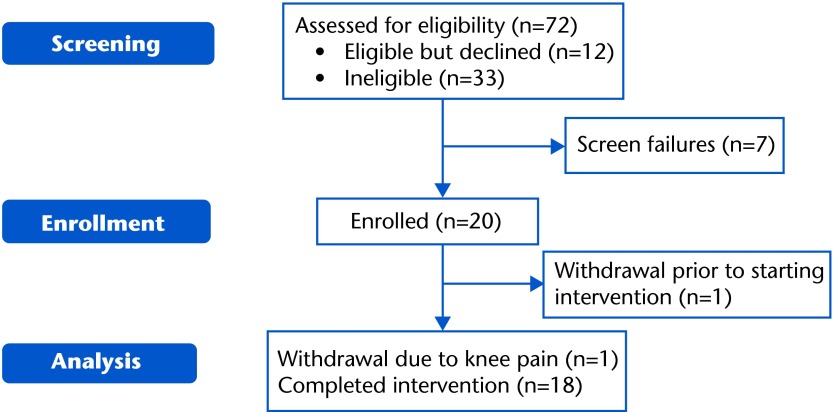
The numbers of participants who consented, enrolled, and completed the study are shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Fig. 1). Screen failures after consent were secondary to exclusion criteria (n=3), uncontrolled hyperglycemia (n=1; referred to a physician for follow-up), or difficulty with the exercise test (n=3; 2 participants had angina and ST segment depression during the cool-down phase of the exercise test, and all 3 participants were referred to a physician for follow-up and were not enrolled in the study). After enrollment, 2 participants withdrew—1 before starting the intervention secondary to a new open wound that required hospitalization and 1 voluntary withdrawal after starting the intervention because of knee pain.
Figure 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram.
Participant demographics and medical information are shown in Table 2. Although dose and type of medication may have changed during the study, only one participant reported stopping or starting a medication—from using insulin at the beginning of the study to not using insulin at the end of the study. On the basis of standard criteria,28 DPN was identified as confirmed in the majority of participants (n=12; 66.7%), probable in 4 participants (22%), and possible in 2 participants (11%).
Table 2.
Participant Characteristics (N=18)a
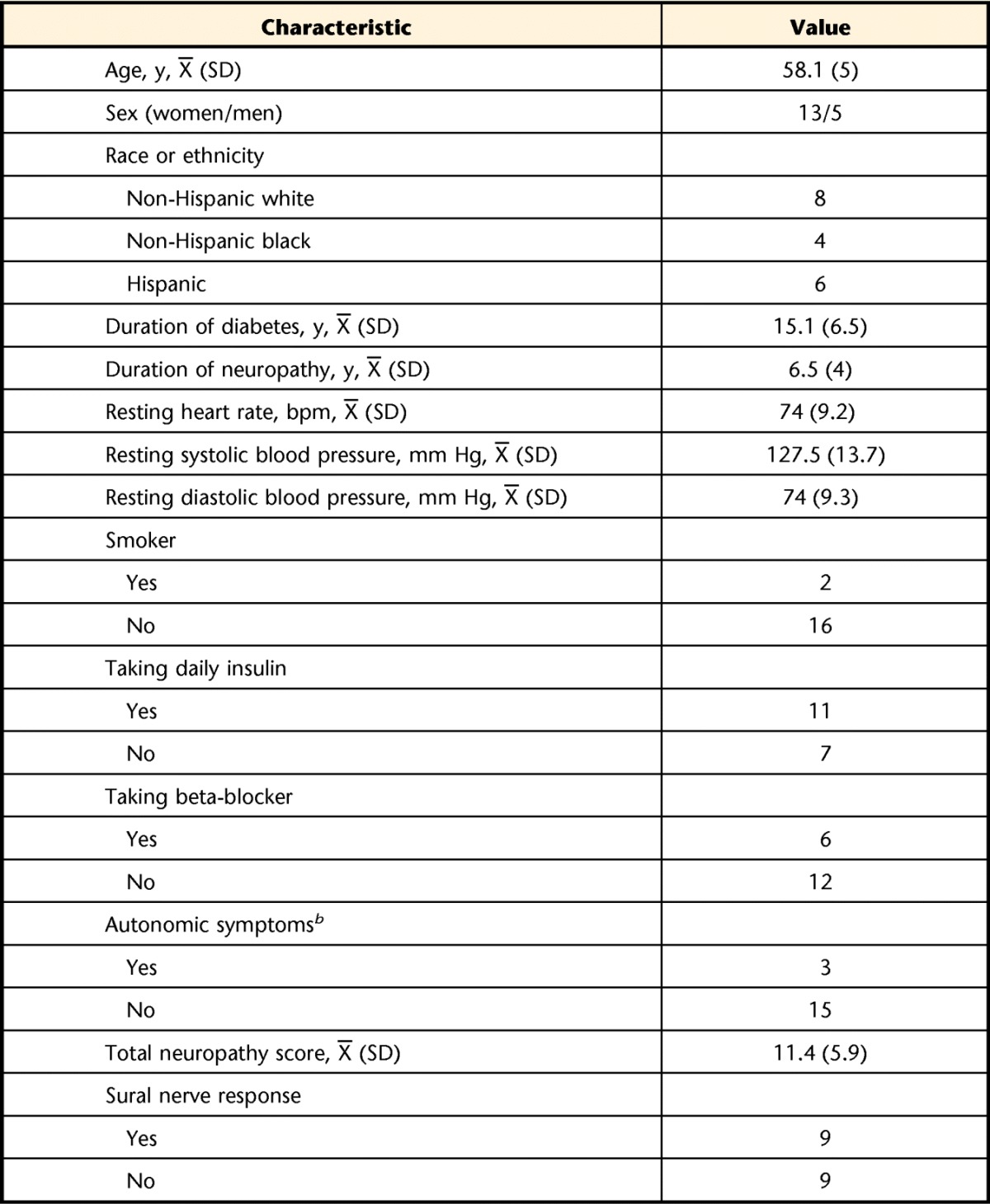
Values are reported as numbers of participants, unless otherwise indicated. bpm=beats per minute.
b For autonomic symptoms, “yes” was recorded if the participant reported the presence of an autonomic symptom (eg, fainting, impotence, constipation, or loss of bladder or bowel control).
Attendance at the 48 scheduled exercise sessions was 67.8% (SD=19.9%, range=33.3%–93.8%). On several occasions, participants missed the scheduled supervised sessions and reported exercising at home instead. Inclusion of the home exercise sessions increased the mean exercise adherence to 76.6% (SD=16.5%, range=33.3%–95.8%).
Summary of AEs
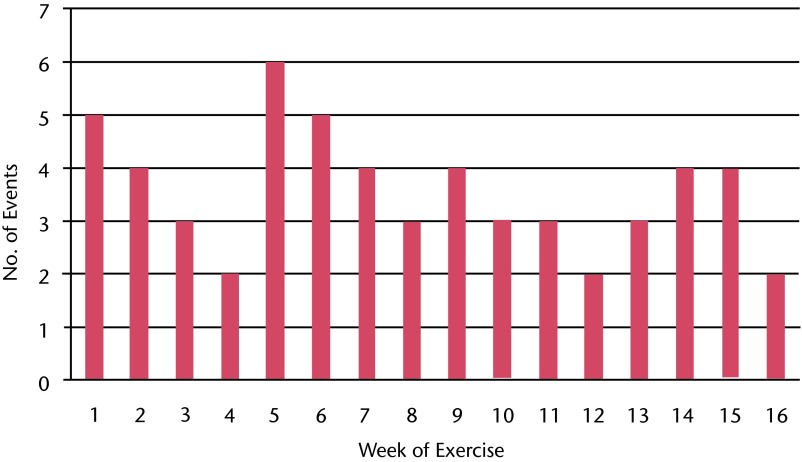
No serious AEs occurred in enrolled participants as a result of the study procedures, and no unanticipated AEs occurred as a result of the study procedures. A total of 57 grade 2 AEs (clinical symptoms that required minimal, noninvasive intervention) occurred in the 18 participants during the 16-week study intervention. A frequency distribution of these events is shown in Figure 2. Slightly more than half of the events (56%) occurred in the first 8 weeks of the 16-week exercise program.
Figure 2.
Frequency histogram of exercise-related adverse events that required intervention during each week of the 16-week exercise program.
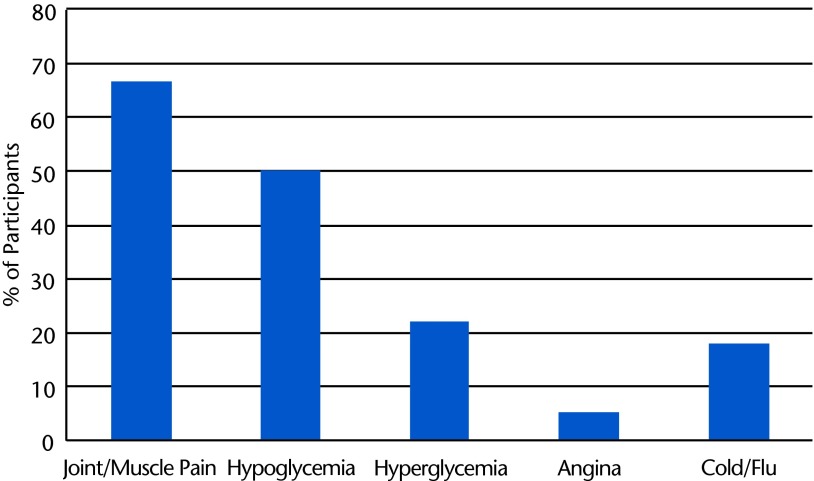
The following anticipated AEs occurred and were attributed to study participation (percentages of participants are shown in Fig. 3):
Joint or muscle pain that affected exercise duration or attendance
Significant hypoglycemia (blood glucose level of <70 mg/dL)
Chest pain and shortness of breath (occurred in one participant during peak effort in the final graded exercise test of the postintervention testing session; no electrocardiography changes were noted; the participant was referred to a cardiologist, but no cardiac diagnosis was identified)
Figure 3.
Frequency histogram of the percentages of participants reporting various types of adverse events.
Other AEs that were not related to the study procedures but that did affect participation in exercise occurred. For example, significant hyperglycemia (blood glucose level of >300 mg/dL) was not caused by exercise but resulted in close monitoring during exercise for safety, and cold or flu symptoms occasionally caused cancellation of exercise sessions. One participant developed a foot ulcer after wearing new shoes in the community (not during study exercise), and a wound care specialist approved continued, non-weight-bearing exercise in a recumbent stepper with an offloading boot. Several other issues were noted by the study team and resulted in a physician referral; these included significant foot swelling (n=1), hyperglycemia with a positive urine ketone test result (n=1), and significant hypertension at rest (n=1).
Changes in Outcomes
A comparison of means before and after the intervention is shown in Table 3. Missing data occurred for a variety of reasons:
General fatigue (n=17): 1 missing data point because of an incomplete form
V̇o2peak (n=16): 2 missing data points because final tests were not completed due to illness
Sleep quality (n=16): 2 missing data points because of incomplete forms
Bone density (n=17): 1 missing data point because of report error
Plasma analysis (n=16): 2 missing insulin results and 1 missing all plasma results because of laboratory errors
FMD (n=17): 1 missing data point because the test was not completed
Table 3.
Outcome Measures Before and After the Exercise Interventiona
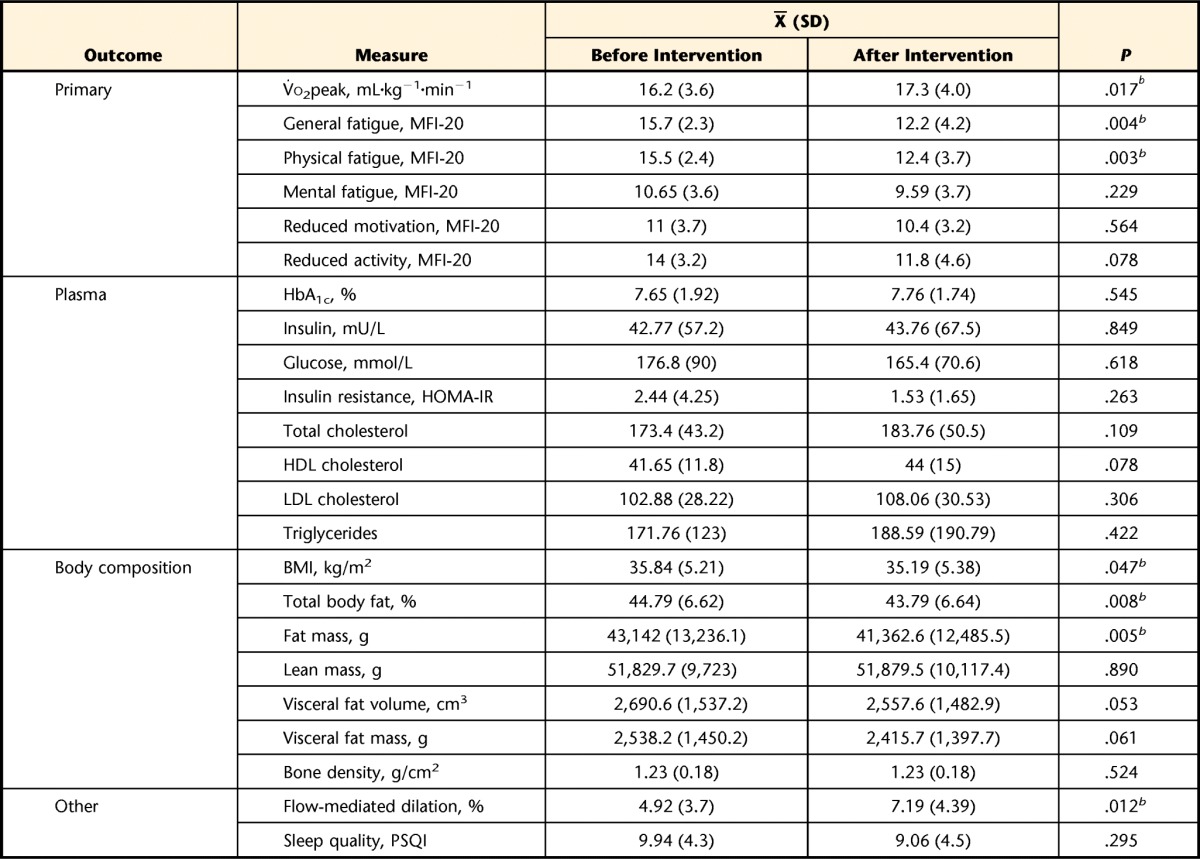
V̇o2peak=peak oxygen uptake during a graded exercise test, MFI-20=Multidimensional Fatigue Inventory, HbA1c=glycated hemoglobin A1c, HOMA-IR=homeostasis model assessment–insulin resistance index, HDL=high-density lipoprotein, LDL=low-density lipoprotein, BMI=body mass index, PSQI=Pittsburgh Sleep Quality Index.
b P≤.05, as determined with a 2-tailed paired t test.
Significant improvements were found in the following outcomes (with 95% confidence intervals for changes): general fatigue (95% CI=−1.3, −5.3), physical fatigue (95% CI=−1.2, −5.0), V̇o2 peak (95% CI=0.2, 1.9), total body fat (95% CI=−0.3, −1.7), fat mass (95% CI=−616.2, −2,938.7), and peripheral blood flow (95% CI=0.6, 4.0). No significant changes were noted in the other outcome measures.
A comparison of changes in primary outcomes between participants who completed less than 80% of the exercise sessions (n=10) and those who completed greater than 80% of the exercise sessions (n=8) revealed a significant difference between the groups for both V̇o2peak and general fatigue. A significant correlation was noted between a change in general fatigue and percentage of exercise sessions attended (r= −.72, P=.01), as shown in the eFigure, but not between a change in V̇o2peak and percentage of exercise sessions attended (r=.3, P=.253).
Discussion
The present study demonstrated that a moderate-intensity, 16-week supervised aerobic exercise intervention was feasible in people with DPN, in the sense that 18 of 20 enrolled participants (90%) completed the study and no serious AEs occurred as a result of the study. Our findings are consistent with those of a systematic review of 47 randomized controlled trials of exercise in people with diabetes: dropout rates of less than 20% and adherence rates of greater than 75%.48 However, a surprising number of minor AEs that required intervention from our physical therapist team occurred throughout the 16-week intervention in the present study.
It is possible that the use of the V̇o2 reserve formula to calculate target HR may have led to an intensity of aerobic exercise that was too high and that contributed to the high frequency of AEs. However, the target HR was used as a goal to work toward rather than a minimum threshold for effort, and a moderate RPE level was used to supplement the target HR. As AEs were highest upon initiation of the exercise program and during progression from 50% to 70% of V̇o2 reserve, it is possible that increases in session intensity and duration may have been too fast. Although the progression of exercise was consistent with published recommendations for people with diabetes,31 a slower progression may have been more tolerable for the participants, who were sedentary and had low aerobic fitness. Only a small number of previous studies on exercise in people with diabetes reported nonserious AEs, but those that were reported most commonly included hypoglycemia, cardiovascular events, and musculoskeletal injuries.48,49
The data raise a concern for people who may be exercising at home without supervision and may not consistently check their blood glucose or blood pressure. Complaints of joint or muscle pain were also common barriers to exercise adherence and were not necessarily neuropathic in origin. Delayed-onset muscle soreness is common after the initiation of a new exercise regimen,50 but the complaints occurred throughout the 16-week intervention in the present study.
The results of the present study supported our hypotheses that aerobic exercise would improve self-reported fatigue and increase V̇o2peak in people with DPN. These findings add to a body of emerging research that has shown promising effects of exercise on various outcomes in people with DPN.18–22 However, this exploratory preliminary study had significant limitations, especially the small sample size, lack of a control or comparison group, and multiple dependent variables, which may increase the type I error rate. Although there was a potential for bias with regard to the hypothesized changes because of a lack of masking and participant expectations for change, several of the outcome measures (eg, V̇o2peak, body composition, and plasma measures) were obtained by people who were not part of the study team, and standardized procedures were used for all tests to minimize bias.
Despite these limitations, to our knowledge the present study is the first to report the effects of exercise on fatigue outcomes in people with DPN. Improvements in self-reported fatigue were found for the general and physical fatigue subscales but not for the measures of mental fatigue, motivation, or activity. At baseline, the values for general and physical fatigue in our participants (15.7 and 15.5, respectively) were higher than those previously reported (12–12.7) in people with diabetes,36,51 perhaps reflecting the influence of neuropathy in our participants. The changes in these outcomes in our participants after exercise (−3.5 and −3.1, respectively) not only were statistically significant but also were above the minimal clinically important differences (2.0–2.1) established for these measures in people with cancer-related fatigue.34
At baseline, the participants in the present study had V̇o2peak values well below the “very poor” threshold of 19.3 mL·kg−1·min−1, indicating that they were less fit than the lowest 1% of people who were 50 to 59 years old.29 Maximum V̇o2 levels below the 20th percentile are associated with an increased risk of death from all causes, indicating that our participants were at high risk if they did not improve their fitness.29 These extremely low values may reflect the difference between V̇o2peak in the present study and the comparison maximum V̇o2. All of our participants achieved a respiratory exchange ratio of greater than 1.0 and an RPE of greater than or equal to 17 of 20 (indicating “very hard” effort) during each exercise test. However, 15 of the 18 participants (83.3%) did not achieve 85% of the age-predicted maximal HR during the baseline and follow-up tests. Diabetes is known to blunt the HR response during exercise,29 and the use of beta-blockers in 6 participants (33.3%) likely contributed to this finding, along with fatigue and muscle endurance.
Although no other studies have reported changes in V̇o2peak after exercise specifically in people with DPN, the effects of exercise in people with type 2 diabetes have been extensively studied. A large-scale randomized controlled trial in people with type 2 diabetes showed a mean increase in V̇o2peak of 1.73 mL·kg−1·min−1 after 6 months of aerobic training.8 This change was greater than that in the present study, possibly reflecting the longer duration of the intervention. In other studies, submaximal tests were used to estimate aerobic fitness,14,49,52 making a comparison of values difficult.
It was interesting that a significant relationship was found between a change in general fatigue and exercise attendance but not between a change in aerobic fitness and exercise attendance, as would be expected. Fatigue is a complex construct with multiple potential contributing factors, including sleep quality, pain, obesity, and depression.26,53 The increased response of fatigue to the dose of exercise may have been due to the cumulative effects of exercise on these factors and may reflect motivational influences as well.
We did note significant decreases in body fat and increases in peripheral vascular blood flow after the exercise intervention. Other studies have identified peripheral vascular dysfunction as an important contributor to decreased exercise capacity in people with type 2 diabetes54,55 and have reported that aerobic exercise can improve FMD,56,57 but these parameters were not previously studied or reported specifically in people with DPN.
The baseline HbA1c values in our participants indicated that many would not be considered to have “well-controlled” diabetes, as the average HbA1c value was above the recommended goal of 7%.15 People with HbA1c values of greater than 7% have been found to have higher levels of oxidative stress and inflammatory markers than people who have diabetes and HbA1c values of less than 7%.58 The lack of change in HbA1c in our participants after exercise was surprising and inconsistent with a large body of research on the change in HbA1c with exercise in people with diabetes.59 However, this parameter has not been extensively studied in people with DPN.
In conclusion, the present study provides new support for improvements in fatigue, fitness, and several potential contributing factors after a supervised exercise intervention in people with DPN. However, a future large-scale randomized controlled trial is needed to fully assess the effects of exercise on these outcomes. Physical therapists can play an important role in the prescription of exercise intensity on the basis of the initial level of aerobic fitness and may need to provide close monitoring and education to address the anticipated AEs related to glycemic control and musculoskeletal pain as people who are sedentary and have DPN begin an exercise program.
The Bottom Line
What do we already know about this topic?
Diabetes can cause damage to nerves in the legs, with resulting pain, numbness, and difficulty walking. Exercise is essential to improve the health of people with diabetes, but people with diabetic nerve damage may have more difficulty exercising.
What new information does this study offer?
The study participants, all with diabetic nerve damage, were able to complete the exercise program without serious problems. However, several minor problems with pain or blood sugar values occurred. After completing the exercise program, the participants had decreased fatigue and other benefits.
If you're a patient, what might these findings mean for you?
Exercise may be helpful if you have diabetes with nerve damage in your legs. However, it may be important to receive guidance from a physical therapist or other health care professional when starting an exercise program.
Footnotes
Dr Kluding, Dr Singh, Ms D'Silva, Mr Yoo, Dr Dimachkie, and Dr Wright provided concept/idea/research design. Dr Kluding, Mr Yoo, Dr Billinger, Dr LeMaster, Dr Dimachkie, Ms Herbelin, and Dr Wright provided writing. Dr Kluding, Dr Pasnoor, Dr Singh, Ms D'Silva, Mr Yoo, and Dr Billinger provided data collection. Dr Kluding, Ms D'Silva, Mr Yoo, Dr Billinger, Dr LeMaster, Dr Dimachkie, and Dr Wright provided data analysis. Dr Kluding, Ms D'Silva, Mr Yoo, and Dr Wright provided project management. Dr Kluding provided fund procurement, participants, and institutional liaisons. Dr Kluding, Dr Billinger, Ms Herbelin, and Dr Wright provided facilities/equipment. Mr Yoo provided administrative support. Dr Pasnoor, Dr Singh, Ms D'Silva, Dr Billinger, Dr LeMaster, Dr Dimachkie, and Dr Wright provided consultation (including review of the manuscript before submission). The authors acknowledge the essential contributions of Bill Hendry, CES, and the medical monitors and nursing staff at the Clinical and Translational Science Unit for exercise testing; Jason-Flor Sisante for vascular scans; Christian Pearson for nerve testing; and Katherine Martin, Gurpreet Singh, Ali Bani-Ahmed, Chelsea Kufahl, Kayla Lingenfelter, and Sara Nelson for supervising the exercise sessions and assisting with data entry and data management.
Institutional review board approval for the project was received from the Human Subjects Committee at the University of Kansas Medical Center.
The primary findings were presented as a poster presentation at the American Diabetes Association's 74 Scientific Sessions; June 13–17, 2014; San Francisco, California. Preliminary results of this project also were presented at the 2014 Combined Sections Meeting of the American Physical Therapy Association; February 4–6, 2014; Las Vegas, Nevada.
This work was supported by Clinical and Translational Science Award (CTSA) program grants from the National Center for Advancing Translational Sciences (NCATS), awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research UL1TR000001 and TL1TR000120 (for M.Y.). Support also was provided by grants T32HD057850 (for L.J.D.) and K01HD067318 (for S.A.B.) from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NCATS, or the National Institutes of Health.
ClinicalTrials.gov trial registration: NCT01764373.
References
- 1. Pasnoor M, Dimachkie M, Kluding P, Barohn RJ. Diabetic neuropathy, part 1: overview and symmetric phenotypes. Neurol Clin. 2013;31:425–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson JK. Factors associated with falls in older patients with diffuse polyneuropathy. J Am Geriatr Soc. 2002;50:1767–1773. [DOI] [PubMed] [Google Scholar]
- 3. Thurman D, Stevens J, Rao J. Practice parameter: assessing patients in a neurology practice for risk of falls (an evidence-based review). Neurology. 2008;70:473–479. [DOI] [PubMed] [Google Scholar]
- 4. Mueller MJ, Minor SD, Sahrmann SA, et al. Differences in the gait characteristics of patients with diabetes and peripheral neuropathy compared with age-matched controls. Phys Ther. 1994;74:299–308. [DOI] [PubMed] [Google Scholar]
- 5. Simoneau GG, Ulbrecht JS, Derr JA, et al. Postural instability in patients with diabetic sensory neuropathy. Diabetes Care. 1994;17:1411–1421. [DOI] [PubMed] [Google Scholar]
- 6. van Sloten TT, Savelberg HH, Duimel-Peeters IG, et al. Peripheral neuropathy, decreased muscle strength and obesity are strongly associated with walking in persons with type 2 diabetes without manifest mobility limitations. Diabetes Res Clin Pract. 2011;91:32–39. [DOI] [PubMed] [Google Scholar]
- 7. Boule NG, Haddad E, Kenny GP, et al. Effect of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta-analysis of controlled clinical trials. JAMA. 2001;286:1218–1227. [DOI] [PubMed] [Google Scholar]
- 8. Larose J, Sigal RJ, Boule NG, et al. Effect of exercise training on physical fitness in type II diabetes mellitus. Med Sci Sports Exerc. 2010;42:1439–1447. [DOI] [PubMed] [Google Scholar]
- 9. Sigal RJ, Kenny GP, Boule NG, et al. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann Intern Med. 2007;147:357–369. [DOI] [PubMed] [Google Scholar]
- 10. Davidson LE, Hudson R, Kilpatrick K, et al. Effects of exercise modality on insulin resistance and functional limitation in older adults: a randomized controlled trial. Arch Intern Med. 2009;169:122–131. [DOI] [PubMed] [Google Scholar]
- 11. Knauf MT, Koltyn KF. Exercise-induced modulation of pain in adults with and without painful diabetic neuropathy. J Pain. 2014;15:656–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association. Standards of medical care in diabetes—2008. Diabetes Care. 2008;31(suppl 1):S12–S54. [DOI] [PubMed] [Google Scholar]
- 13. American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care. 2010;33(suppl 1):S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. LeMaster JW, Mueller MJ, Reiber GE, et al. Effect of weight-bearing activity on foot ulcer incidence in people with diabetic peripheral neuropathy: Feet First randomized controlled trial. Phys Ther. 2008;88:1385–1398. [DOI] [PubMed] [Google Scholar]
- 15. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(suppl 1):S14–S80. [DOI] [PubMed] [Google Scholar]
- 16. Colberg SR, Sigal RJ, Fernhall B, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association joint position statement. Diabetes Care. 2010;33:147–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mueller MJ, Tuttle LJ, LeMaster JW, et al. Weight-bearing versus nonweight-bearing exercise for persons with diabetes and peripheral neuropathy: a randomized controlled trial. Arch Phys Med Rehabil. 2013;94:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kluding P, Pasnoor M, Singh R, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012;26:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Smith AG, Russell JW, Feldman EL, et al. Lifestyle intervention for pre-diabetic neuropathy. Diabetes Care. 2006;29:1294–1299. [DOI] [PubMed] [Google Scholar]
- 20. Song C, Petrofsky J, Lee S, et al. Effects of an exercise program on balance and trunk proprioception in older adults with diabetic neuropathies. Diabetes Technol Ther. 2011;13:803–811. [DOI] [PubMed] [Google Scholar]
- 21. Richardson JK, Sandman D, Vela S. A focused exercise regimen improves clinical measures of balance in patients with peripheral neuropathy. Arch Phys Med Rehabil. 2001;82:205–209. [DOI] [PubMed] [Google Scholar]
- 22. Allet L, Armand S, De Bie R, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kruse RL, LeMaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “Feet First” randomized controlled trial. Phys Ther. 2010;90:1568–1579. [DOI] [PubMed] [Google Scholar]
- 24. Sudore RL, Karter AJ, Huang ES, et al. Symptom burden of adults with type 2 diabetes across the disease course: Diabetes & Aging Study. J Gen Intern Med. 2012;27:1674–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh R, Kluding P. Fatigue and related factors in people with type 2 diabetes. Diabetes Educ. 2013;39:320–326. [DOI] [PubMed] [Google Scholar]
- 26. Tseng BY, Billinger SA, Gajewski BJ, Kluding PM. Exertion fatigue and chronic fatigue are two distinct constructs in people post-stroke. Stroke. 2010;41:2908–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mayer CJ, Steinman L, Williams B, et al. Developing a Telephone Assessment of Physical Activity (TAPA) questionnaire for older adults. Prev Chronic Dis. 2008;5:A24. [PMC free article] [PubMed] [Google Scholar]
- 28. Dyck PJ, Albers JW, Andersen H, et al. Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab Res Rev. 2011;27:620–628. [DOI] [PubMed] [Google Scholar]
- 29. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 8th ed Philadelphia, PA: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 30. Chen MJ, Fan X, Moe ST. Criterion-related validity of the Borg ratings of perceived exertion scale in healthy individuals: a meta-analysis. J Sports Sci. 2002;20:873–899. [DOI] [PubMed] [Google Scholar]
- 31. Colberg SR, Sigal RJ. Prescribing exercise for individuals with type 2 diabetes: recommendations and precautions. Phys Sportsmed. 2011;39:13–26. [DOI] [PubMed] [Google Scholar]
- 32. Common Terminology Criteria for Adverse Events v3.0 (CTCAE). Published August 9, 2006. Available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Accessed October 10, 2014.
- 33. Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. [DOI] [PubMed] [Google Scholar]
- 34. Purcell A, Fleming J, Bennett S, et al. Determining the minimal clinically important difference criteria for the Multidimensional Fatigue Inventory in a radiotherapy population. Support Care Cancer. 2010;18:307–315. [DOI] [PubMed] [Google Scholar]
- 35. Lasselin J, Layé S, Barreau JB, et al. Fatigue and cognitive symptoms in patients with diabetes: relationship with disease phenotype and insulin treatment. Psychoneuroendocrinology. 2012;37:1468–1478. [DOI] [PubMed] [Google Scholar]
- 36. Lasselin J, Layé S, Dexpert S, et al. Fatigue symptoms relate to systemic inflammation in patients with type 2 diabetes. Brain Behav Immun. 2012;26:1211–1219. [DOI] [PubMed] [Google Scholar]
- 37. Billinger SA, Loudon JK, Gajewski BJ. Validity of a total body recumbent stepper exercise test to assess cardiorespiratory fitness. J Strength Cond Res. 2008;22:1556–1562. [DOI] [PubMed] [Google Scholar]
- 38. Buysse DJ, Reynolds CF, III, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatr Res. 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 39. Buysse DJ, Yu L, Moul DE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33:781–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cole JC, Motivala SJ, Buysse DJ, et al. Validation of a 3-factor scoring model for the Pittsburgh Sleep Quality Index in older adults. Sleep. 2006;29:112–116. [DOI] [PubMed] [Google Scholar]
- 41. Nishiyama T, Mizuno T, Kojima M, et al. Criterion validity of the Pittsburgh Sleep Quality Index and Epworth Sleepiness Scale for the diagnosis of sleep disorders. Sleep Med. 2014;15:422–429. [DOI] [PubMed] [Google Scholar]
- 42. Spira AP, Beaudreau SA, Stone KL, et al. Reliability and validity of the Pittsburgh Sleep Quality Index and the Epworth Sleepiness Scale in older men. J Gerontol A Biol Sci Med Sci. 2012;67:433–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sarafidis PA, Lasaridis AN, Nilsson PM, et al. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens. 2007;21:709–716. [DOI] [PubMed] [Google Scholar]
- 44. Billinger SA, Mattlage AE, Ashenden AL, et al. Aerobic exercise in subacute stroke improves cardiovascular health and physical performance. J Neurol Phys Ther. 2012;36:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. [DOI] [PubMed] [Google Scholar]
- 46. Thijssen DH, Black M, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300:H2–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. National Institutes of Health. Neuromuscular diseases. NINDS Common Data Elements Web site. 2014. Available at: http://www.commondataelements.ninds.nih.gov/NMD.aspx#tab=Data_Standards Accessed October 10, 2014.
- 48. Umpierre D, Ribeiro PA, Kramer CK, et al. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2011;305:1790–1799. [DOI] [PubMed] [Google Scholar]
- 49. Herbert RD, de Noronha M, Kamper SJ. Stretching to prevent or reduce muscle soreness after exercise. Cochrane Database Syst Rev. 2011;7:CD004577. [DOI] [PubMed] [Google Scholar]
- 50. Balducci S, Zanuso S, Cardelli P, et al. Effect of high- versus low-intensity supervised aerobic and resistance training on modifiable cardiovascular risk factors in type 2 diabetes: the Italian Diabetes and Exercise Study (IDES). PLoS One. 2012;7:E49297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fritschi C, Quinn L, Hacker ED, et al. Fatigue in women with type 2 diabetes. Diabetes Educ. 2012;38:662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Balducci S, Zanuso S, Cardelli P, et al. Changes in physical fitness predict improvements in modifiable cardiovascular risk factors independently of body weight loss in subjects with type 2 diabetes participating in the Italian Diabetes and Exercise Study (IDES). Diabetes Care. 2012;35:1347–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schmidt ME, Chang-Claude J, Seibold P, et al. Determinants of long-term fatigue in breast cancer survivors: results of a prospective patient cohort study. Psychooncology. 2014. May 17 [Epub ahead of print]. doi: 10.1002/pon.3581. [DOI] [PubMed] [Google Scholar]
- 54. Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord. 2013;14:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bauer TA, Reusch JE, Levi M, Regensteiner JG. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care. 2007;30:2880–2885. [DOI] [PubMed] [Google Scholar]
- 56. Kwon HR, Min KW, Ahn HJ, et al. Effects of aerobic exercise vs. resistance training on endothelial function in women with type 2 diabetes mellitus. Diabetes Metab J. 2011;35:364–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Maiorana A, O'Driscoll G, Cheetham C, et al. The effect of combined aerobic and resistance exercise training on vascular function in type 2 diabetes. J Am Coll Cardiol. 2001;38:860–866. [DOI] [PubMed] [Google Scholar]
- 58. Gohel MG, Chacko AN. Serum GGT activity and hsCRP level in patients with type 2 diabetes mellitus with good and poor glycemic control: an evidence linking oxidative stress, inflammation and glycemic control. J Diabetes Metab Disord. 2013;12:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3:CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]