Abstract
The influences and mechanisms of the physiology, rupture and reconstruction of the anterior cruciate ligament (ACL) on kinematics and clinical outcomes have been investigated in many biomechanical and clinical studies over the last several decades. The knee is a complex joint with shifting contact points, pressures and axes that are affected when a ligament is injured. The ACL, as one of the intra-articular ligaments, has a strong influence on the resulting kinematics. Often, other meniscal or ligamentous injuries accompany ACL ruptures and further deteriorate the resulting kinematics and clinical outcomes. Knowing the surgical options, anatomic relations and current evidence to restore ACL function and considering the influence of concomitant injuries on resulting kinematics to restore full function can together help to achieve an optimal outcome.
Keywords: Biomechanics, Anterior cruciate ligament, Joint pressure, Anterior cruciate ligament rupture, Graft fixation, Anterior cruciate ligament reconstruction
Core tip: This review of literature summarizes the influences and mechanisms of the physiology, rupture and reconstruction of the anterior cruciate ligament on kinematics and clinical outcomes. The major focuses are on the resulting joint kinematics after rupture and reconstruction and on biomechanics of graft fixation.
INTRODUCTION
Biomechanics is one major key to the function, stability and aging process of joints. The knee is a major and complex joint. Its stability and motion are basically controlled by ligaments such as the anterior cruciate ligament (ACL)[1].
The ACL is a central ligament of the knee. The main functional role of the ACL is to provide stability against anterior tibial translation (ATT) and internal rotation. An acute ACL rupture is a common orthopedic trauma, with an estimated incidence of 78 per 100000 persons and a mean age of 32 years in Sweden and an estimated incidence of up to 84 per 100000 persons in the United States[2,3].
A common and frequent injury mechanism is non-contact combined valgus- and internal-rotation trauma[2,4]. Therefore, ACL injuries are often associated with other ligamentous injuries, such as a (partial) rupture of the medial collateral ligament (MCL) or the menisci. In addition, compression of the lateral condyle with a bone bruise or chondral lesion is often associated with the injury due to the valgus trauma. Persistent instability of the knee may be associated with long-term degenerative lesions. Surgical treatment of the ACL in the context of other injured structures and reconstruction of the intact joint kinematics are suggested to be the keys to a good clinical outcome[5,6].
ANATOMY
The ACL has its origin at the medial area of the lateral femoral condyle and inserts into the center of the eminentia of the tibia plateau next to the anterior horn of the lateral meniscus. The structure of the ACL has been described as two functional bundles: The anteromedial (AM) and the posterolateral (PL) bundle[7]. These two bundles have been associated with different roles in anteroposterior and complex-rotational stabilization of the joint[8-10]. The femoral origin was described as oval shaped with a longitudinal diameter of 18 mm and a width of approximately 11 mm[11,12]. The AM bundle is inserted deep in the intercondylar notch directly in front of the intercondylar line and the edge of the chondral bone. The femoral insertion of the PL bundle is located at anterior of the AM bundle, also bordering the edge of the chondral bone. The tibial insertion of the AM bundle is located close to the anterior horn of the lateral meniscus at approximately the first 30% mark of a virtual sagittal line crossing the tibial plateau, while the PL bundle inserts slightly posterolateral to the AM bundle at approximately 44% of the virtual sagittal line[9,13]. The anatomy of the ACL and functional bundles has been biomechanically evaluated in many studies[8-10,14], and surgical techniques have evolved as a result. Due to the oval shape of the femoral condyles, the position of the joint axis varies during flexion in the sagittal plane[15]. The oval/flat structure of the ACL plays an important role in stabilizing the knee joint under different flexion angles and, therefore, compensating for shifting knee flexion axes[10,14,16]. The PL bundle has been shown to have particular stabilizing effects on the anteroposterior and rotational forces in near-to-extension positions of less than 30°, whereas the AM bundle becomes tensioned and functional at higher flexion angles[8,10,16]. Nevertheless, in a recent study, Kondo et al[14] found that the influence and reciprocal relationship of an insulated AM or PL bundle tear might have been overestimated. Recently, the existing knowledge of ACL anatomy was enhanced by a landmark anatomical study. Śmigielski et al[17] analyzed the detailed anatomical ACL structure of 111 human cadaveric knees. They found that femoral insertion and midsubstance of the ACL were thinner than previously assumed, and they determined a width of 11-17 mm and a thickness of only approximately 3 mm (Figure 1). Additionally, the tibial insertion site was recently anatomically analyzed by Siebold et al[18] and described as a “C”-shaped structure.
Figure 1.
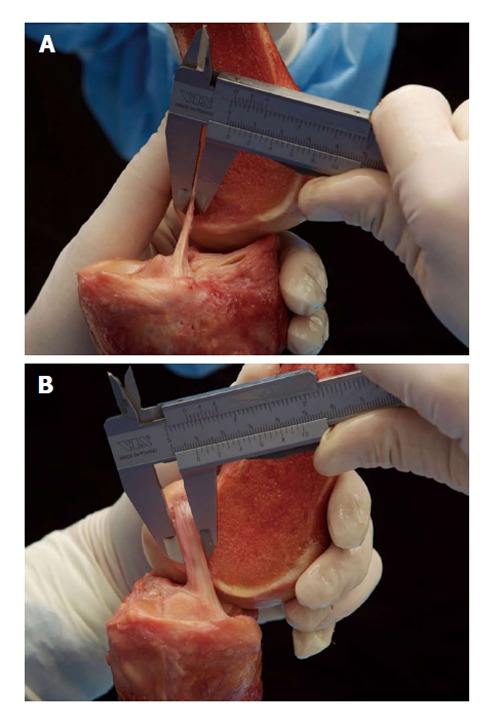
Śmigielski et al[17] measuring the thickness (A) and width (B) of the “ribbon-like” midsubstance of the anterior cruciate ligament.
In addition to collagen fibers, nerves and mechanoreceptors are integrated within the ACL and play an important role in the proprioception of the joint[19,20]. Nevertheless, there are more proprioceptive elements involved around the knee, such as other ligaments, muscles and the capsule.
KINEMATICS
Anterior tibial translation
In the intact knee, the ACL provides essential support for ATT and internal rotation. This functional role must be achieved at the base of the described anatomic insertion sites of the ACL, the complex oval-like shape of the condyles, and the tensile characteristics of the ligament[15]. In extension, the ATT is low, with a maximum 2 mm scope, and provides support while standing. In flexion angles and when applying an external anteroposterior load, the ATT may increase up to 3 mm when walking and up to 5.5 mm under the anterior tibial load[1].
When the ACL is ruptured or dissected, the ATT increases by up to 10 to 15 mm at 30° of knee flexion under 134 N of anterior load[10,14,21]. Robotic/universal force-moment sensor (UFS) testing systems have been able to quantify passive ATT under the anterior tibial load in cadaveric knees at different flexion angles without being influenced by active muscle forces. Without these muscle forces, the highest increase in ATT was found between 15° and 40° of flexion. Clinically, ATT is often tested at different flexion angles. Stress radiographs, KT-1000 or rolimeters can help quantify the clinically observed ATT. The ischiocrural muscle group induces flexion by connecting the tuber ischiadicum with the proximal crus (pes anserinus tibia and fibular head). These muscle groups show a greater than 70° posterior force vector at 90° of knee flexion, which actively stabilizes against ATT (Figure 2). Considering these ligamentous and muscular kinematics, the ATT may therefore be clinically evaluated most accurately at near to extension angles (15° to 30°).
Figure 2.
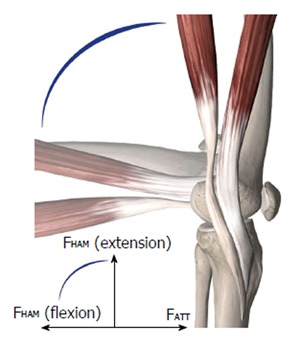
Semitendinosus and gracilis muscles with their insertions. At 90° of flexion, the forces of the hamstrings (FHAM) are opposed to the anterior tibial translation (ATT) forces (FATT).
Cutting-edge studies have demonstrated an important role of the two functional ACL bundles for ATT and pivot shift at different flexion angles. In cadaveric studies, a stabilizing role for the PL bundle in controlling the ATT at near-to-extension angles was found[8,10,16]. The AM bundle seemed to have more influence in controlling higher flexion angles. Nevertheless, the suggested reciprocal relation of the two bundles is controversial and is still being discussed. A recent human cadaveric study by Kondo et al[14] showed different result s than prior studies: They reported that partial tears of the AM or the PL bundle showed a nonsignificant and less-than-expected increase of ATT. According to previous studies[8,10,16], the tension of the PL bundle, as indicated by the distances of the bundle’s insertion sites, was increased at near-to-extension angles. Nevertheless, unlike others, Kondo et al[14] found that AM bundle tensioning did not increase with growing flexion angles but stayed rather constant between 0° and 120° of flexion (Figure 3). Therefore, they concluded that when detecting a clinically unstable ATT in an examination of the knee, more than a partial (one-bundle) rupture of the ACL has to be assumed.
Figure 3.
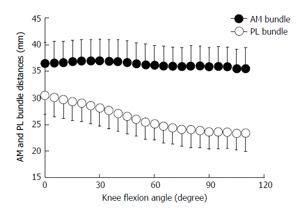
Distance between the femoral and tibial attachments of the anteromedial and posterolateral bundles of the anterior cruciate ligament during knee flexion (mean/SD) from Kondo et al[14]. AM: Anteromedial; PL: Posterolateral.
Rotational instability and pivot shift testing
Considering the anatomy of the ACL, its main structure has a complex diagonal route through the knee (anteroposterior and horizontal mediolateral fibers), which is almost reciprocal to the posterior cruciate ligament fibers[22]. The role of the mediolateral ACL fibers might be versatile. In the intact knee, these fibers resist a complex internal tibial rotational force. Although a correlation between increased internal tibial rotation and deficient ACL seems obvious, the internal tibial rotation increases by less than 4°, from up to 30° of internal rotation in the intact knee[15], when the ACL is completely ruptured[14,22], as other collateral ligaments are also important stabilizers against internal rotation[23].
Although the resulting effect on isolated internal rotation stability seems small, the rotational axis of the knee alters from the center to a medial position near the pars intermedia of the internal meniscus when the ACL is ruptured[24]. As a consequence, the movement in the lateral compartment increases (Figure 4).
Figure 4.
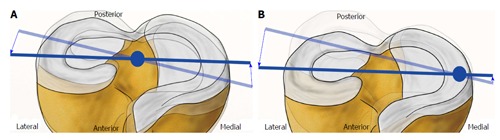
Shift of the center of the rotatory axis from the center/eminentia in an anterior cruciate ligament-intact knee (A) to a medial position (B). Modified from Amis et al[24].
Kanamori et al[25] determined that both medial and lateral collateral ligaments compensate for in situ forces against internal rotation when the ACL is ruptured. Based on the effects of the medialized center of rotation with a subsequent increase of motion radius of the lateral compartment after an ACL rupture, the in situ forces of the posterolateral ligamentous structures increase by up to 413% at 15° of knee flexion[25]. The influence of an ACL rupture on near-to-extension pivoting kinematics can be clinically assessed by the pivot shift test.
The pivot shift test is an established and valid clinical test for examining the ACL’s influence on complex rotational instability when ACL is ruptured[26]. It not only is a dynamic knee instability test with a high specificity for ACL ruptures but also is influenced by active muscle forces and by an inter-observer bias[27]. Near extension the combined internal rotatory and valgus loads induces an anterolateral tibial subluxation in the ACL-deficient knee. With ongoing flexion, the collateral ligament apparatus and the iliotibial tractus reach tension at approximately 30° and retract the subluxated tibia[26]. Biomechanically, the pivot shift examination can be simulated in vitro with a robotic/UFS system by applying combined internal rotatory (4-5 Nm) and valgus (10 Nm) loads while measuring the resulting anterolateral tibial translation[8,21,22,28,29]. Several in vitro studies have demonstrated a correlation between a positive pivot shift phenomenon and a deficiency of horizontal (e.g., PL bundle) fibers, as in the case of a steep PL-to-High-AM reconstruction[8,10,16]. Clinically, the pivot shift phenomenon may be difficult to detect, especially in a situation of pain from an acute ACL tear and subsequent contraction of muscles, which can inhibit the subluxation phenomenon. Additionally, due to improving technologies, such as navigation and sensors, new techniques and tools have been developed to quantify clinical pivot shift kinematics[30-32]. Although the pivot shift seems more complicated to evaluate compared to the ATT, studies report its strong correlation to clinical outcomes[33]. In a clinical study of 63 patients with a follow-up at 5 to 9 years after ACL reconstruction surgery, Jonsson et al[34] found a positive correlation of the pivot shift phenomenon and osteoarthritic changes. Kocher et al[35] investigated the relationship between clinical assessment and different outcome parameter scores after ACL reconstruction surgery. While there was no correlation with investigated parameters regarding a positive ATT, a positive pivot shift was detected to be a significant predictor of satisfaction, giving way, difficulty in different types of activity, overall knee function, sports participation, and inferior Lysholm score.
Joint pressure
After an ACL rupture with a subsequent shift of the rotatory axis and increased instability, an altered intra-articular cartilage pressure seems obvious[24]. Several biomechanical in vivo and in vitro studies have found decreased total joint pressure after an ACL rupture by pressure-sensitive sensors or electromyography driven model[36-38]. As a possible consequence of decreased joint pressure, the knee’s flexion movement after an ACL rupture is also reduced[37,39]. The complex kinematics of the knee joint and the altered strain of other ligamentous or cartilage structures suggest the importance of distinguishing the joint pressure in terms of its intra-articular sector, loading or passive condition and respective flexion angle[15]. In the ACL-deficient knee, there is a shift of the rotatory axis to the medial compartment with slightly increased freedom of motion[22,24]. Li et al[40] determined the tibiofemoral contact points during a one-legged lunge at different flexion angles in an in vivo study (Figure 5). They found out that there was a significant shift of the contact points on the tibial surface after an ACL rupture, most of them at flexion angles close to 15°. In the medial compartment, the contact points altered to a more posterior and more lateral position toward the intercondylar eminentia. In the lateral compartment, a lateralization of contact points was also observed but without alteration in the anteroposterior axis. While total patellofemoral joint pressure and the pressure of the medial patellofemoral compartment decrease after an ACL rupture, the lateral patellofemoral joint pressure increases at higher flexion angles above 60°[36]. In a human cadaveric study, Imhauser et al[41] measured increased tibiofemoral contact forces in the posterior medial and lateral compartments under axial load and simulated Lachman and pivot shift tests after an ACL rupture (Figure 5).
Figure 5.
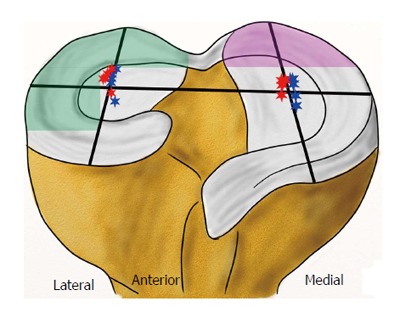
Lateral shift of femorotibial contact points at various flexion angles with intact anterior cruciate ligament (blue stars) and deficient anterior cruciate ligament (red stars) - modified from Li et al[40]. Increased contact stress after an anterior cruciate ligament rupture in the posterior lateral compartment during pivot shifting (green area) and in the posterior medial compartment during Lachman testing (violet area) - modified from Imhauser et al[41].
In situ forces and tensile characteristics
In situ forces of the ACL have been calculated by Morrison[42-44]. For normal walking, in situ forces of 169 N were observed. When descending stairs, increased in situ forces of 445 N were determined, which Morrison explained by the complementary effects of knee extensor muscles. Woo et al[45] performed tensile testing of young human cadaveric femur-ACL-tibia complexes and determined an ultimate load to failure of 2160 (± 157) N with a linear stiffness of 242 (± 28) N/mm.
INFLUENCE OF ACL RUPTURE ON OTHER STRUCTURES OF THE KNEE
A non-contact combined valgus- and internal-rotation trauma of the knee is described as one of the most frequent mechanisms for an ACL rupture, as it may occur in pivoting sports such as in soccer or handball[2,4]. This complex rotational trauma mechanism indicates that other structures should be examined to determine whether they were injured when an ACL rupture is suspected. The incidences of other accompanying injuries with the trauma mechanism and with altered kinematics of ACL rupture are detailed below.
MCL
MCL rupture is a frequent possible result of the valgus-stress component of a typical ACL trauma. The co-incidence of MCL rupture was reported in every fifth case when the ACL was ruptured[4,46]. An overseen and maintained medial instability after ACL reconstruction may result in inferior kinematics and persistent giving way, despite sufficient anteroposterior and pivot-rotational stability. In a clinical study, Zaffagnini et al[47] found a persistent valgus instability after three years post ACL surgery and conservative treatment of a chronic MCL grade II rupture, but there was no difference in anteroposterior stability and in clinical outcome scores after three years compared to a surgically treated MCL. In another prospective randomized clinical study, Halinen et al[48] also found equal stability and clinical outcome scores after operative or nonoperative treatment of concomitant MCL grade III lesions and combined ACL reconstruction in acute cases. Nevertheless, due to persistent instability, a higher risk for secondary graft failure after ACL reconstruction and for osteoarthritis is still discussed. Medial muscles, especially vastus medialis muscle, are considered to provide active stabilizing effects on medial instability. Therefore, alternative tendon grafts, other than the medial-inserting m. semitendinosus tendon, can be considered for ACL reconstruction, but the exact influence of the hamstring muscles has not yet been investigated.
Medial meniscus
Medial meniscus concomitant injuries are reported in 18%-54% of cases[4,46,49,50] and have a comorbidity to acute and chronic ACL tears of up to 90%[51]. A possible cause for this correlation in acute valgus- and internal-rotational trauma could be the mechanism of sudden medial instability with subsequent anteroposterior and rotational shear forces when the ACL ruptures. This shear trauma might even be enhanced by an accompanying MCL rupture[4]. With persistent ACL instability, a shifted rotatory axis[24] and contact points[40] result in an altered flexion path of the medial femoral condyle and increase the risk for secondary injuries of the medial meniscus[51,52]. Shear forces deriving from anteroposterior instability of the tibia will either extend the anterior displacement of the medial meniscus by up to 15 mm or result in compression forces on the meniscus[10,14,21,24]. Decreased passive joint pressure within the medial compartment[37,38] and combined instability seem to result in increased impact forces[41] under stress, e.g., walking. Allen et al[53] determined an increased peak yield of 50 N at 60° of flexion. The correlation of biomechanical influences between the ACL and the medial meniscus has been investigated in biomechanical human cadaveric studies. After dissection of the ACL and the medial meniscus, an increased ATT in contrast to a dissected ACL with an intact medial meniscus was found[29,54].
Lateral meniscus
Lateral meniscus injuries are reported with concomitant rates of 17%-51%[4,46,49,50], which is slightly less frequent than medial meniscus tears in cases of acute ACL rupture but also often caused by the typical valgus-internal-rotation trauma mechanism. The risk for a lateral meniscus injury seems to correlate with a coexisting lateral bone bruise, which is indicated on an MRI[46]. Additionally, like the medial meniscus, the lateral meniscus provides significant stability. A deficient lateral meniscal root evokes pivot-rotational instability of the knee in cadaveric studies[54,55].
Lateral collateral ligament
Lateral collateral ligament (LCL) concomitant injuries are rare and may be caused by anterolateral knee luxation injuries[46]. Nevertheless, the LCL is an important stabilizer for the joint. Zantop et al[56] have shown in a biomechanical study that a standalone ACL reconstruction cannot restore intact knee kinematics if the LCL is also deficient. Increased pivot-rotational anterolateral instability after an ACL rupture has promoted anatomical investigations of the anterolateral corner[57]. Parsons et al[58] found that the anterolateral ligament (ALL) provides stability against internal rotation at flexion angles greater than 35°. It has been proposed that a deficient ALL may correlate with a positive pivot shift sign. In addition, other structures of the anterolateral corner, such as the iliotibial tractus with its connecting Kaplan fibers, the lateral retinaculum and the lateral capsule, are suggested to influence pivot-rotational stability[57]. Additionally, it is currently being discussed whether the ALL becomes insufficient over time in cases of chronic ACL insufficiency[59].
Secondary osteoarthritis
Secondary osteoarthritis is often observed after an ACL rupture: A meta-analysis by Ajuied et al[60] calculated a relative risk of 389 for radiographic osteoarthritic progression after an ACL rupture. There is good evidence for long-term degenerative progression and inferior clinical outcomes after concomitant meniscal and chondral injuries[60,61]. Additionally, persistent pivot-rotational instability after an ACL rupture was found to be a predictor for osteoarthritis and subjective satisfaction[33-35]. Nevertheless, although a positive effect of ACL reconstruction on osteoarthritic progression is proposed, to date there is no evidence for a preventive effect[60,61].
ACL RECONSTRUCTION
ACL reconstruction is currently a topic of intense research, with more than 1940 hits in the Medline database for the term “ACL reconstruction” published within the past 5 years. Nevertheless, the history of ACL reconstruction goes back to the end of the 19th century. In 1895, Robson[62] directly sewed the ACL in place via open medial arthrotomy. In 1903, Lange proposed an alloplastic ACL reconstruction with a silk graft[5]. Grekow described in 1914 the first autologous transplant with a free iliotibial tractus stripe[5]. From that moment, different autologous grafts have been used for transplantation. Brückner used a free patellar tendon graft for an arthroscopically assisted transplantation in 1966[63]. Because of the high harvesting morbidity of the graft, the primary suture regained favor in the 1970s[64,65]. Additionally, new synthetic materials (e.g., Goretex, Carbonates) were used, but, due to complications, these alloplastic grafts, as well as the primary suture, were considered obsolete in the 1990s[66]. Since then, arthroscopically assisted ACL reconstruction with autologous or allogeneic tendon graft has evolved to worldwide acceptance.
Graft choice and graft fixation
Currently, hamstring-tendons (semitendinosus and gracilis), bone-patellar-tendon-bone (BPTB) complexes and quadriceps tendon-bone complexes are frequently used grafts. Tensile characteristics of these grafts are shown to be superior (maximum loads 2977 N[67], 4140 N and 2353 N) to the native femur-ACL-tibia complex (2160 N)[45] with similar stiffness (Table 1)[45,67-73]. Nevertheless, the weakest point of primary graft failure has turned out to be the graft fixation (Table 1). There are many biomechanical studies that evaluate the biomechanical properties of graft fixation. Due to the scarcity of young human donors, animal or old human specimens have often been used, leading to results that may vary from those of typical young ACL reconstruction patients. Still, the dimensions of biomechanical properties range between the calculated in situ forces of the ACL[42-44] and its ultimate failure load[45]. In addition to sufficient failure loads of fixation techniques, fixation of the graft close to the insertion site is suggested to decrease longitudinal (“bungee”) and sagittal (“windshield wiper”) graft movements[66,68]. Furthermore, Oh et al[68] have found significantly increased stiffness when an interference screw was added to an extracortical endobutton fixation (307 N/mm vs 195 N/mm).
Table 1.
Biomechanical properties of tendons, grafts and fixation techniques
| Subject | Maximum load to failure (N) | Stiffness (N/mm) | Ref. |
| Intact ACL (with femur and tibia) | 2160 (± 157) | 242 (± 28) | [45] |
| Two gracilis strands | 1550 (± 369) | 370 (± 108) | [69] |
| Two semitendinosus strands | 2640 (± 320) | 534 (± 76) | [69] |
| Four combined hamstring strands | 4090 (± 295) | 276 (± 204) | [69] |
| 7 mm BPTB | 2238 (± 316) | 327 (± 58) | [67] |
| 10 mm BPTB | 2977 (± 516) | 424/455 (± 57/67) | [67] |
| 15 mm BPTB | 4389 (± 708) | 556 (± 67) | [67] |
| 10 mm QTB | 2353 (± 495) | 621 (± 122) | [70] |
| Interference screw (BPTB) | 683-863 | 76-80 | [71] |
| Interference screw (hamstrings) | 534-925 | 189-315 | [68,72] |
| Endobutton (hamstrings) | 520-1364 | 35-195 | [68,73] |
| Interference screw and endobutton (hamstrings) | 1290-1449 | 307-341 | [68] |
ACL: Anterior cruciate ligament; QTB: Quadriceps-tendon-bone; BPTB: Bone-patellar-tendon-bone.
Beyond biomechanics, clinical studies have shown good and comparable results with semitendinosus, quadriceps and bone-patellar-tendon-bone grafts for ACL reconstructions[74-76]. Thus, it is feasible to make an individual graft choice - for instance, to avoid BPTB grafts when anterior knee pain is at risk (floor tiler) or to avoid semitendinosus grafts when concomitant medial collateral ligament injury exists. Novel minimally invasive surgical harvesting techniques for quadriceps tendons promise less cosmetic burden and more clinical acceptance[77]. In a systematic review of overlapping meta-analyses, Mascarenhas et al[78] concluded that allografts were equal to autografts in terms of rerupture rates and clinical outcomes.
Tunnel positioning and effects on biomechanics
Until the beginning of the 21st century, ACL reconstruction with transtibial drilling of the femoral tunnel was the common surgical technique[66]. As femoral tunnel placement depended on the tibial tunnel placement, it was difficult to aim for the anatomic ACL footprint of the lateral femur condyles through a tibial tunnel. The over-the-top position, with tunnel placement near the deep cartilage margin and the intercondylar roof, was preferred to achieve a good near-to-anatomic reconstruction[66]. From an anatomical point of view, this position corresponds best to a near AM-bundle position. In 2002, Loh et al[79] were possibly the first to transform the known reciprocal relation of ACL bundles in situ forces into a cadaveric ACL reconstruction study. By drilling the femur in the 10 o’clock position, Loh intended to address PL-bundle fibers and evaluated the results against the conventional 11 o’clock over-the-top position. After evaluating resulting kinematics with a UFS/robotic testing system, the 10 o’clock reconstruction resulted in superior stability under combined rotatory loads. Consideration of anatomical ACL insertion sites continues to be a focus of biomechanical investigations. Mismatch reconstruction studies using robotic/UFS testing systems have demonstrated superior kinematics for combined rotatory loads and near-to-extension ATT when fibers of both the PL and AM bundles are reconstructed (Figure 6)[8,16,21,80].
Figure 6.
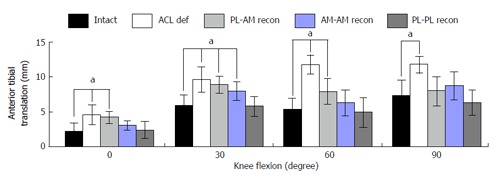
Anterior tibial translation under a simulated pivot shift test (combined 10 Nm valgus and 4 Nm internal rotational load, mean/SD, aP < 0.05) as influenced by anterior cruciate ligament mismatch reconstruction of posterolateral and anteromedial bundles in a human cadaveric study by Herbort et al[8]. AM: Anteromedial; PL: Posterolateral; ACL: Anterior cruciate ligament.
Most authors have concluded that the more horizontal fibers of the reconstructed PL bundle in particular were responsible for stabilizing the joint at near-to-extension angles.
With the “dependent” transtibial drilling technique, accurately and reliably addressing the femoral ACL footprint was very difficult[81,82], although recently a novel modified transtibial technique was introduced that promises better aiming for the native footprint[83]. Nevertheless, the introduction of the anteromedial portal technique allowed “independent” drilling of femoral ACL tunnels and, thus, a method to reliably and visually address the native tibial and femoral ACL footprints[84,85]. Hence, novel anteromedial portal “anatomical” single- and double-bundle ACL reconstruction techniques have been clinically introduced and biomechanically evaluated[21,86-89].
Correlating to the results of the biomechanical mismatch studies[8,16], both anatomic double-bundle and anatomic single-bundle ACL reconstruction techniques resulted in superior kinematics compared to transtibial (“high-AM”) femoral tunnel drilling for ACL reconstruction[21,89,90]. In a human cadaveric study, Bedi et al[91] determined that even an increased graft sized of a “high-AM” misplaced femoral ACL single bundle could not compensate for prime stability in contrast to a centrally placed smaller single bundle. However, in biomechanical studies, no superior kinematics could be found between anatomic double-bundle ACL reconstruction with drilling of an AM and a PL bundle tunnel in contrast to one centrally placed “anatomic” single-bundle ACL reconstruction[21,28,90]. Regardless, biomechanical advantages of “anatomic” double-bundle reconstruction may exist in larger knees: Siebold[92] calculated better coverage of femoral ACL insertion sites larger than 16 mm with the double-bundle technique and good coverage of the femoral ACL footprint for sites smaller than 13 mm with the single-bundle technique.
From a clinical perspective, Chhabra et al[93] and Griffith et al[94] reported less tunnel expansion, decreased instability and fewer incidences of revision surgery after ACL reconstruction using the anteromedial portal technique compared to the transtibial technique. There have been many prospective randomized clinical studies comparing anatomic single- and double-bundle ACL reconstruction[95,96]. Several of the clinical studies have shown minor but significant advantages for the anatomic double-bundle technique. In their meta-analysis, Desai et al[96] included 15 prospective clinical trials and found that 3 of those indicated significant superior anteroposterior stability of the anatomic double-bundle group in contrast to the anatomic single-bundle group. Furthermore, 2 of the included clinical studies resulted in superior pivot-rotational stability[96]. Regarding clinical outcome scores, van Eck et al[95] reported in their meta-analysis of 12 prospective clinical studies that no significantly different parameters of IKDC score, Lysholm score, range of motion and complication rate between anatomic double-bundle and anatomic single-bundle were detected. Despite these good biomechanical and clinical results, the anatomic double-bundle ACL reconstruction is controversial. In contrast to anatomic single-bundle reconstruction, its upfront costs are more expensive[97], and the surgical technique and tunnel revision surgery are considered to be more sophisticated[98].
New trends and concepts in ACL reconstruction surgery
Trying to simulate anatomic ACL insertion sites has resulted in good biomechanical and clinical parameters over the last decade. To mimic the effect of the anatomic double-bundle technique to effectively address both the PL and the AM bundle for femoral tunnel drilling, Herbort et al[99] introduced a rectangular tunnel technique, which is supposed to show better coverage of both bundle insertions with one single, regular-sized transplant. Their biomechanical human cadaveric study resulted in superior ATT stability in contrast to a conventional round-tunnel, single-bundle reconstruction at 0° and 15° of flexion[99]. Sonnery-Cottet et al[59] and Kittl et al[57] reported a combined ACL and ALL reconstruction technique (with tenodesis) to improve the stability of the anterolateral corner in cases of acute or chronic ACL rupture with increased anterolateral rotational instability (Figure 7C) An initial case study of 92 patients resulted in significantly improved pivot-rotational stability after 2 years[59]. However, prospective randomized clinical studies to distinguish the pivot-rotational stabilizing effects between a conventional ACL reconstruction and a combined ACL/ALL reconstruction are still lacking. Primary suture with or without alloplastic augmentation of the ruptured ACL has resulted in inferior clinical outcomes in prospective and retrospective studies[66,100]. The majority of these alloplastic augmentations have been stiff constructs in a nonanatomical position (over-the-top position or extra-articular)[66,100]. However, with the aim to restore proprioceptive properties of the ligament and to avoid the harvesting morbidity of tendon grafts, Eggli et al[101], and Kohl et al[102] have introduced a new method of primary suturing of a traumatic ACL rupture with combined dynamic alloplastic intraligamentous stabilization (Figure 7A). To avoid mechanical fatigue of the augmentation and to adapt to the complex flexion axis of the knee[15], the augmentation cord is placed near-anatomical within or slightly behind the ACL and dynamically suspended by an intraosseous spring mechanism. Initial small-sample clinical studies after suturing with dynamic alloplastic intraligamentous stabilization have shown promising results for primary ACL ruptures and for combined suturing and augmentation of other structures for complex knee dislocation injuries[101,103].
Figure 7.
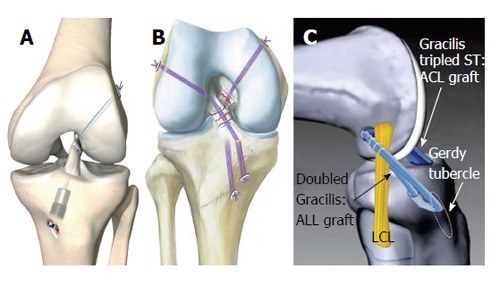
Novel anterior cruciate ligament repair techniques. A: Anterior cruciate ligament (ACL) suture and dynamic augmentation (Ligamys™, copyright Mathys Ltd., Bettlach, Switzerland); B: Ligament bracing (from Heitmann et al[104]); C: ACL reconstruction with anterolateral ligament (ALL) augmentation (from Sonnery-Cottet et al[59]). ST: Semitendinosus, LCL: Lateral collateral ligament.
Heitmann et al[104] described a similar technique with suturing and anatomically placed intraligamentous alloplastic augmentation of torn cruciate ligaments in cases of acute knee dislocation injury (Figure 7B). The authors exclusively recommend the technique for acute knee dislocations injuries of Schenck III and IV types that do not involve a dynamic suspension of the augmentation. An initial clinical study of 20 patients showed satisfying results one year after surgery[104].
The perspective of suturing for preserving autologous graft material and the native ACL has often been considered to reduce morbidity in the past, but the results were not promising[66,100]. For complex ligamentous knee dislocation injuries, there seems to be a good potential for reduced harvesting morbidity and OR time. Additionally, it remains unclear whether a dynamic or a static augmentation suture in a combined cruciate ligament injury was best either for restoring stability or not inducing deficits of range-of-motion or misplacing the physiological axes of the knee. To date, there are no data from prospective randomized controlled studies.
CONCLUSION
The knee is a complex joint with shifting contact points, pressures and axes that are affected when a ligament is injured. The ACL, as one of the intra-articular ligaments, has a strong influence on the resulting kinematics. Often, other meniscal or ligamentous injuries accompany ACL rupture and further deteriorate resulting kinematics and clinical outcomes. Knowing the surgical options, anatomic relations and current evidence to restore ACL function and considering the influence of concomitant injuries on resulting kinematics to restore full function together can help to achieve an optimal outcome.
Footnotes
Supported by A Research Fellowship from the Faculty of Medicine, Westphalian Wilhelms University Muenster to Domnick C.
Conflict-of-interest statement: There are no conflicts of interest to declare.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 5, 2015
First decision: August 16, 2015
Article in press: December 2, 2015
P- Reviewer: Bicanic G, Fukuchi RK, Mugnai R, Seijas R S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
References
- 1.Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58:583–594. [PubMed] [Google Scholar]
- 2.Nordenvall R, Bahmanyar S, Adami J, Stenros C, Wredmark T, Felländer-Tsai L. A population-based nationwide study of cruciate ligament injury in Sweden, 2001-2009: incidence, treatment, and sex differences. Am J Sports Med. 2012;40:1808–1813. doi: 10.1177/0363546512449306. [DOI] [PubMed] [Google Scholar]
- 3.Griffin LY, Albohm MJ, Arendt EA, Bahr R, Beynnon BD, Demaio M, Dick RW, Engebretsen L, Garrett WE, Hannafin JA, Hewett TE, Huston LJ, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II Meeting. 2005. pp. 1512–1532. [DOI] [PubMed] [Google Scholar]
- 4.Bates NA, McPherson AL, Rao MB, Myer GD, Hewett TE. Characteristics of inpatient anterior cruciate ligament reconstructions and concomitant injuries. Knee Surg Sports Traumatol Arthrosc. 2014:Epub ahead of print. doi: 10.1007/s00167-014-3478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhardt C, Jäger A, Schwetlick G, Rauschmann MA. [History of surgery of the anterior cruciate ligament] Orthopade. 2002;31:702–709. doi: 10.1007/s00132-002-0329-6. [DOI] [PubMed] [Google Scholar]
- 6.Zysk SP, Refior HJ. Operative or conservative treatment of the acutely torn anterior cruciate ligament in middle-aged patients. A follow-up study of 133 patients between the ages of 40 and 59 years. Arch Orthop Trauma Surg. 2000;120:59–64. doi: 10.1007/pl00021217. [DOI] [PubMed] [Google Scholar]
- 7.Weber W, Weber E. Mechanics of the Human Walking Apparatus. Springer Verlag: 1992. pp. 75–92. [Google Scholar]
- 8.Herbort M, Lenschow S, Fu FH, Petersen W, Zantop T. ACL mismatch reconstructions: influence of different tunnel placement strategies in single-bundle ACL reconstructions on the knee kinematics. Knee Surg Sports Traumatol Arthrosc. 2010;18:1551–1558. doi: 10.1007/s00167-010-1163-8. [DOI] [PubMed] [Google Scholar]
- 9.Petersen W, Zantop T. Anatomy of the anterior cruciate ligament with regard to its two bundles. Clin Orthop Relat Res. 2007;454:35–47. doi: 10.1097/BLO.0b013e31802b4a59. [DOI] [PubMed] [Google Scholar]
- 10.Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. Am J Sports Med. 2007;35:223–227. doi: 10.1177/0363546506294571. [DOI] [PubMed] [Google Scholar]
- 11.Sasaki N, Ishibashi Y, Tsuda E, Yamamoto Y, Maeda S, Mizukami H, Toh S, Yagihashi S, Tonosaki Y. The femoral insertion of the anterior cruciate ligament: discrepancy between macroscopic and histological observations. Arthroscopy. 2012;28:1135–1146. doi: 10.1016/j.arthro.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 12.Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction. J Bone Joint Surg Am. 1985;67:257–262. [PubMed] [Google Scholar]
- 13.Siebold R, Ellert T, Metz S, Metz J. Tibial insertions of the anteromedial and posterolateral bundles of the anterior cruciate ligament: morphometry, arthroscopic landmarks, and orientation model for bone tunnel placement. Arthroscopy. 2008;24:154–161. doi: 10.1016/j.arthro.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Kondo E, Merican AM, Yasuda K, Amis AA. Biomechanical analysis of knee laxity with isolated anteromedial or posterolateral bundle-deficient anterior cruciate ligament. Arthroscopy. 2014;30:335–343. doi: 10.1016/j.arthro.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Freeman MA, Pinskerova V. The movement of the normal tibio-femoral joint. J Biomech. 2005;38:197–208. doi: 10.1016/j.jbiomech.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Kato Y, Maeyama A, Lertwanich P, Wang JH, Ingham SJ, Kramer S, Martins CQ, Smolinski P, Fu FH. Biomechanical comparison of different graft positions for single-bundle anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21:816–823. doi: 10.1007/s00167-012-1951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Śmigielski R, Zdanowicz U, Drwięga M, Ciszek B, Ciszkowska-Łysoń B, Siebold R. Ribbon like appearance of the midsubstance fibres of the anterior cruciate ligament close to its femoral insertion site: a cadaveric study including 111 knees. Knee Surg Sports Traumatol Arthrosc. 2015;23:3143–3150. doi: 10.1007/s00167-014-3146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siebold R, Schuhmacher P, Fernandez F, Śmigielski R, Fink C, Brehmer A, Kirsch J. Flat midsubstance of the anterior cruciate ligament with tibial “C”-shaped insertion site. Knee Surg Sports Traumatol Arthrosc. 2015;23:3136–3142. doi: 10.1007/s00167-014-3058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bali K, Dhillon MS, Vasistha RK, Kakkar N, Chana R, Prabhakar S. Efficacy of immunohistological methods in detecting functionally viable mechanoreceptors in the remnant stumps of injured anterior cruciate ligaments and its clinical importance. Knee Surg Sports Traumatol Arthrosc. 2012;20:75–80. doi: 10.1007/s00167-011-1526-9. [DOI] [PubMed] [Google Scholar]
- 20.Iwasa J, Ochi M, Uchio Y, Adachi N, Kawasaki K. Decrease in anterior knee laxity by electrical stimulation of normal and reconstructed anterior cruciate ligaments. J Bone Joint Surg Br. 2006;88:477–483. doi: 10.1302/0301-620X.88B4.17186. [DOI] [PubMed] [Google Scholar]
- 21.Kondo E, Merican AM, Yasuda K, Amis AA. Biomechanical comparison of anatomic double-bundle, anatomic single-bundle, and nonanatomic single-bundle anterior cruciate ligament reconstructions. Am J Sports Med. 2011;39:279–288. doi: 10.1177/0363546510392350. [DOI] [PubMed] [Google Scholar]
- 22.Diermann N, Schumacher T, Schanz S, Raschke MJ, Petersen W, Zantop T. Rotational instability of the knee: internal tibial rotation under a simulated pivot shift test. Arch Orthop Trauma Surg. 2009;129:353–358. doi: 10.1007/s00402-008-0681-z. [DOI] [PubMed] [Google Scholar]
- 23.Kim YH, Purevsuren T, Kim K, Oh KJ. Contribution of posterolateral corner structures to knee joint translational and rotational stabilities: a computational study. Proc Inst Mech Eng H. 2013;227:968–975. doi: 10.1177/0954411913490456. [DOI] [PubMed] [Google Scholar]
- 24.Amis AA, Bull AMJ, Lie DTT. Biomechanics of rotational instability and anatomic anterior cruciate ligament reconstruction. Oper Tech Orthop. 2005;15:29–35. [Google Scholar]
- 25.Kanamori A, Sakane M, Zeminski J, Rudy TW, Woo SL. In-situ force in the medial and lateral structures of intact and ACL-deficient knees. J Orthop Sci. 2000;5:567–571. doi: 10.1007/s007760070007. [DOI] [PubMed] [Google Scholar]
- 26.Galway HR, MacIntosh DL. The lateral pivot shift: a symptom and sign of anterior cruciate ligament insufficiency. Clin Orthop Relat Res. 1980;(147):45–50. [PubMed] [Google Scholar]
- 27.Bull A, Amis AA. The pivot-shift phenomenon: a clinical and biomechanical perspective. The Knee. 1998;5:141–158. [Google Scholar]
- 28.Goldsmith MT, Jansson KS, Smith SD, Engebretsen L, LaPrade RF, Wijdicks CA. Biomechanical comparison of anatomic single- and double-bundle anterior cruciate ligament reconstructions: an in vitro study. Am J Sports Med. 2013;41:1595–1604. doi: 10.1177/0363546513487065. [DOI] [PubMed] [Google Scholar]
- 29.Lorbach O, Kieb M, Domnick C, Herbort M, Weyers I, Raschke M, Engelhardt M. Biomechanical evaluation of knee kinematics after anatomic single- and anatomic double-bundle ACL reconstructions with medial meniscal repair. Knee Surg Sports Traumatol Arthrosc. 2015;23:2734–2741. doi: 10.1007/s00167-014-3071-9. [DOI] [PubMed] [Google Scholar]
- 30.Araujo PH, Ahlden M, Hoshino Y, Muller B, Moloney G, Fu FH, Musahl V. Comparison of three non-invasive quantitative measurement systems for the pivot shift test. Knee Surg Sports Traumatol Arthrosc. 2012;20:692–697. doi: 10.1007/s00167-011-1862-9. [DOI] [PubMed] [Google Scholar]
- 31.Pearle AD, Kendoff D, Musahl V, Warren RF. The pivot-shift phenomenon during computer-assisted anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2009;91 Suppl 1:115–118. doi: 10.2106/JBJS.H.01553. [DOI] [PubMed] [Google Scholar]
- 32.Lorbach O, Kieb M, Brogard P, Maas S, Pape D, Seil R. Static rotational and sagittal knee laxity measurements after reconstruction of the anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 2012;20:844–850. doi: 10.1007/s00167-011-1635-5. [DOI] [PubMed] [Google Scholar]
- 33.Ayeni OR, Chahal M, Tran MN, Sprague S. Pivot shift as an outcome measure for ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2012;20:767–777. doi: 10.1007/s00167-011-1860-y. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson H, Riklund-Ahlström K, Lind J. Positive pivot shift after ACL reconstruction predicts later osteoarthrosis: 63 patients followed 5-9 years after surgery. Acta Orthop Scand. 2004;75:594–599. doi: 10.1080/00016470410001484. [DOI] [PubMed] [Google Scholar]
- 35.Kocher MS. Relationships Between Objective Assessment of Ligament Stability and Subjective Assessment of Symptoms and Function After Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2004;32:629–634. doi: 10.1177/0363546503261722. [DOI] [PubMed] [Google Scholar]
- 36.Tajima G, Iriuchishima T, Ingham SJ, Shen W, van Houten AH, Aerts MM, Shimamura T, Smolinski P, Fu FH. Anatomic double-bundle anterior cruciate ligament reconstruction restores patellofemoral contact areas and pressures more closely than nonanatomic single-bundle reconstruction. Arthroscopy. 2010;26:1302–1310. doi: 10.1016/j.arthro.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 37.Gardinier ES, Manal K, Buchanan TS, Snyder-Mackler L. Altered loading in the injured knee after ACL rupture. J Orthop Res. 2012;31:458–464. doi: 10.1002/jor.22249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khandha A, Gardinier E, Capin J, Manal K, Snyder-Mackler L, Buchanan T. Do decreased medial compartment contact forces and loading asymmetries exist after anterior cruciate ligament reconstruction and rehabilitation? - a 5 year follow-up study. Osteoarthritis Cartilage. 2014;22:S103. [Google Scholar]
- 39.Hart JM, Ko JW, Konold T, Pietrosimone B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clin Biomech (Bristol, Avon) 2010;25:277–283. doi: 10.1016/j.clinbiomech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88:1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 41.Imhauser C, Mauro C, Choi D, Rosenberg E, Mathew S, Nguyen J, Ma Y, Wickiewicz T. Abnormal tibiofemoral contact stress and its association with altered kinematics after center-center anterior cruciate ligament reconstruction: an in vitro study. Am J Sports Med. 2013;41:815–825. doi: 10.1177/0363546512475205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison JB. The mechanics of the knee joint in relation to normal walking. J Biomech. 1970;3:51–61. doi: 10.1016/0021-9290(70)90050-3. [DOI] [PubMed] [Google Scholar]
- 43.Dargel J, Gotter M, Mader K, Pennig D, Koebke J, Schmidt-Wiethoff R. Biomechanics of the anterior cruciate ligament and implications for surgical reconstruction. Strategies Trauma Limb Reconstr. 2007;2:1–12. doi: 10.1007/s11751-007-0016-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morrison JB. Function of the knee joint in various activities. Biomed Eng. 1969;4:573–580. [PubMed] [Google Scholar]
- 45.Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19:217–225. doi: 10.1177/036354659101900303. [DOI] [PubMed] [Google Scholar]
- 46.Yoon KH, Yoo JH, Kim KI. Bone contusion and associated meniscal and medial collateral ligament injury in patients with anterior cruciate ligament rupture. J Bone Joint Surg Am. 2011;93:1510–1518. doi: 10.2106/JBJS.J.01320. [DOI] [PubMed] [Google Scholar]
- 47.Zaffagnini S, Bonanzinga T, Marcheggiani Muccioli GM, Giordano G, Bruni D, Bignozzi S, Lopomo N, Marcacci M. Does chronic medial collateral ligament laxity influence the outcome of anterior cruciate ligament reconstruction?: a prospective evaluation with a minimum three-year follow-up. J Bone Joint Surg Br. 2011;93:1060–1064. doi: 10.1302/0301-620X.93B8.26183. [DOI] [PubMed] [Google Scholar]
- 48.Halinen J, Lindahl J, Hirvensalo E, Santavirta S. Operative and nonoperative treatments of medial collateral ligament rupture with early anterior cruciate ligament reconstruction: a prospective randomized study. Am J Sports Med. 2006;34:1134–1140. doi: 10.1177/0363546505284889. [DOI] [PubMed] [Google Scholar]
- 49.Forkel P, Reuter S, Sprenker F, Achtnich A, Herbst E, Imhoff A, Petersen W. Different patterns of lateral meniscus root tears in ACL injuries: application of a differentiated classification system. Knee Surg Sports Traumatol Arthrosc. 2015;23:112–118. doi: 10.1007/s00167-014-3467-6. [DOI] [PubMed] [Google Scholar]
- 50.Gadeyne S, Besse JL, Galand-Desme S, Lerat JL, Moyen B. [Analysis of meniscal lesions accompanying anterior cruciate ligament tears: A retrospective analysis of 156 patients] Rev Chir Orthop Reparatrice Appar Mot. 2006;92:448–454. doi: 10.1016/s0035-1040(06)75831-7. [DOI] [PubMed] [Google Scholar]
- 51.Jiang W, Gao SG, Li KH, Luo L, Li YS, Luo W, Lei GH. Impact of Partial and complete rupture of anterior cruciate ligament on medial meniscus: A cadavaric study. Indian J Orthop. 2012;46:514–519. doi: 10.4103/0019-5413.101040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krych AJ, Pitts RT, Dajani KA, Stuart MJ, Levy BA, Dahm DL. Surgical repair of meniscal tears with concomitant anterior cruciate ligament reconstruction in patients 18 years and younger. Am J Sports Med. 2010;38:976–982. doi: 10.1177/0363546509354055. [DOI] [PubMed] [Google Scholar]
- 53.Allen CR, Wong EK, Livesay GA, Sakane M, Fu FH, Woo SL. Importance of the medial meniscus in the anterior cruciate ligament-deficient knee. J Orthop Res. 2000;18:109–115. doi: 10.1002/jor.1100180116. [DOI] [PubMed] [Google Scholar]
- 54.Musahl V, Citak M, O’Loughlin PF, Choi D, Bedi A, Pearle AD. The effect of medial versus lateral meniscectomy on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2010;38:1591–1597. doi: 10.1177/0363546510364402. [DOI] [PubMed] [Google Scholar]
- 55.Shybut TB, Vega CE, Haddad J, Alexander JW, Gold JE, Noble PC, Lowe WR. Effect of lateral meniscal root tear on the stability of the anterior cruciate ligament-deficient knee. Am J Sports Med. 2015;43:905–911. doi: 10.1177/0363546514563910. [DOI] [PubMed] [Google Scholar]
- 56.Zantop T, Schumacher T, Schanz S, Raschke MJ, Petersen W. Double-bundle reconstruction cannot restore intact knee kinematics in the ACL/LCL-deficient knee. Arch Orthop Trauma Surg. 2010;130:1019–1026. doi: 10.1007/s00402-010-1081-8. [DOI] [PubMed] [Google Scholar]
- 57.Kittl C, Weiler A, Amis AA. Anterolateral rotatory instability. Arthroskopie. 2014;27:170–176. [Google Scholar]
- 58.Parsons EM, Gee AO, Spiekerman C, Cavanagh PR. The biomechanical function of the anterolateral ligament of the knee. Am J Sports Med. 2015;43:669–674. doi: 10.1177/0363546514562751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sonnery-Cottet B, Thaunat M, Freychet B, Pupim BH, Murphy CG, Claes S. Outcome of a Combined Anterior Cruciate Ligament and Anterolateral Ligament Reconstruction Technique With a Minimum 2-Year Follow-up. Am J Sports Med. 2015;43:1598–1605. doi: 10.1177/0363546515571571. [DOI] [PubMed] [Google Scholar]
- 60.Ajuied A, Wong F, Smith C, Norris M, Earnshaw P, Back D, Davies A. Anterior cruciate ligament injury and radiologic progression of knee osteoarthritis: a systematic review and meta-analysis. Am J Sports Med. 2014;42:2242–2252. doi: 10.1177/0363546513508376. [DOI] [PubMed] [Google Scholar]
- 61.Abermann E, Hoser C, Benedetto KP, Hepperger C, Fink C. Development of osteoarthrosis after anterior cruciate ligament rupture. Arthroskopie. 2015;28:26–30. [Google Scholar]
- 62.Robson AW. VI. Ruptured Crucial Ligaments and their Repair by Operation. Ann Surg. 1903;37:716–718. [PMC free article] [PubMed] [Google Scholar]
- 63.Wirth CJ, Artmann M, J ger M, Refior HJ. Plastic reconstruction of old anterior cruciate ligament ruptures by the Brueckner procedure. Arch orthop Unfall-Chir. 1974;78:362–73. doi: 10.1007/BF00415817. [DOI] [PubMed] [Google Scholar]
- 64.Feagin JA, Curl WW. Isolated tear of the anterior cruciate ligament: 5-year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 65.Odensten M, Lysholm J, Gillquist J. Suture of fresh ruptures of the anterior cruciate ligament. A 5-year follow-up. Acta Orthop Scand. 1984;55:270–272. doi: 10.3109/17453678408992354. [DOI] [PubMed] [Google Scholar]
- 66.Fu FH, Bennett CH, Ma CB, Menetrey J, Lattermann C. Current trends in anterior cruciate ligament reconstruction. Part II. Operative procedures and clinical correlations. Am J Sports Med. 2000;28:124–130. doi: 10.1177/03635465000280010801. [DOI] [PubMed] [Google Scholar]
- 67.Cooper DE, Deng XH, Burstein AL, Warren RF. The strength of the central third patellar tendon graft. A biomechanical study. Am J Sports Med. 1993;21:818–823; discussion 823-824. doi: 10.1177/036354659302100610. [DOI] [PubMed] [Google Scholar]
- 68.Oh YH, Namkoong S, Strauss EJ, Ishak C, Hecker AT, Jazrawi LM, Rosen J. Hybrid femoral fixation of soft-tissue grafts in anterior cruciate ligament reconstruction using the EndoButton CL and bioabsorbable interference screws: a biomechanical study. Arthroscopy. 2006;22:1218–1224. doi: 10.1016/j.arthro.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 69.Hamner DL, Brown CH, Steiner ME, Hecker AT, Hayes WC. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg Am. 1999;81:549–557. doi: 10.2106/00004623-199904000-00013. [DOI] [PubMed] [Google Scholar]
- 70.Stäubli HU, Schatzmann L, Brunner P, Rincón L, Nolte LP. Quadriceps tendon and patellar ligament: cryosectional anatomy and structural properties in young adults. Knee Surg Sports Traumatol Arthrosc. 1996;4:100–110. doi: 10.1007/BF01477262. [DOI] [PubMed] [Google Scholar]
- 71.Kousa P, Järvinen TL, Kannus P, Järvinen M. Initial fixation strength of bioabsorbable and titanium interference screws in anterior cruciate ligament reconstruction. Biomechanical evaluation by single cycle and cyclic loading. Am J Sports Med. 2001;29:420–425. doi: 10.1177/03635465010290040601. [DOI] [PubMed] [Google Scholar]
- 72.Kousa P, Järvinen TL, Vihavainen M, Kannus P, Järvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part I: femoral site. Am J Sports Med. 2003;31:174–181. doi: 10.1177/03635465030310020401. [DOI] [PubMed] [Google Scholar]
- 73.Höher J, Scheffler S, Weiler A. Graft choice and graft fixation in PCL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2003;11:297–306. doi: 10.1007/s00167-003-0408-1. [DOI] [PubMed] [Google Scholar]
- 74.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlén L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42:1049–1057. doi: 10.1177/0363546514526139. [DOI] [PubMed] [Google Scholar]
- 75.Slone HS, Romine SE, Premkumar A, Xerogeanes JW. Quadriceps tendon autograft for anterior cruciate ligament reconstruction: a comprehensive review of current literature and systematic review of clinical results. Arthroscopy. 2015;31:541–554. doi: 10.1016/j.arthro.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 76.Sajovic M, Strahovnik A, Dernovsek MZ, Skaza K. Quality of life and clinical outcome comparison of semitendinosus and gracilis tendon versus patellar tendon autografts for anterior cruciate ligament reconstruction: an 11-year follow-up of a randomized controlled trial. Am J Sports Med. 2011;39:2161–2169. doi: 10.1177/0363546511411702. [DOI] [PubMed] [Google Scholar]
- 77.Fink C, Herbort M, Abermann E, Hoser C. Minimally invasive harvest of a quadriceps tendon graft with or without a bone block. Arthrosc Tech. 2014;3:e509–e513. doi: 10.1016/j.eats.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mascarenhas R, Erickson BJ, Sayegh ET, Verma NN, Cole BJ, Bush-Joseph C, Bach BR. Is there a higher failure rate of allografts compared with autografts in anterior cruciate ligament reconstruction: a systematic review of overlapping meta-analyses. Arthroscopy. 2015;31:364–372. doi: 10.1016/j.arthro.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 79.Loh JC, Fukuda Y, Tsuda E, Steadman RJ, Fu FH, Woo SL. Knee stability and graft function following anterior cruciate ligament reconstruction: Comparison between 11 o’clock and 10 o’clock femoral tunnel placement. 2002 Richard O’Connor Award paper. Arthroscopy. 2003;19:297–304. doi: 10.1053/jars.2003.50084. [DOI] [PubMed] [Google Scholar]
- 80.Driscoll MD, Isabell GP, Conditt MA, Ismaily SK, Jupiter DC, Noble PC, Lowe WR. Comparison of 2 femoral tunnel locations in anatomic single-bundle anterior cruciate ligament reconstruction: a biomechanical study. Arthroscopy. 2012;28:1481–1489. doi: 10.1016/j.arthro.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 81.Kopf S, Forsythe B, Wong AK, Tashman S, Anderst W, Irrgang JJ, Fu FH. Nonanatomic tunnel position in traditional transtibial single-bundle anterior cruciate ligament reconstruction evaluated by three-dimensional computed tomography. J Bone Joint Surg Am. 2010;92:1427–1431. doi: 10.2106/JBJS.I.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piasecki DP, Bach BR, Espinoza Orias AA, Verma NN. Anterior cruciate ligament reconstruction: can anatomic femoral placement be achieved with a transtibial technique? Am J Sports Med. 2011;39:1306–1315. doi: 10.1177/0363546510397170. [DOI] [PubMed] [Google Scholar]
- 83.Youm YS, Cho SD, Lee SH, Youn CH. Modified transtibial versus anteromedial portal technique in anatomic single-bundle anterior cruciate ligament reconstruction: comparison of femoral tunnel position and clinical results. Am J Sports Med. 2014;42:2941–2947. doi: 10.1177/0363546514551922. [DOI] [PubMed] [Google Scholar]
- 84.Harner CD, Honkamp NJ, Ranawat AS. Anteromedial portal technique for creating the anterior cruciate ligament femoral tunnel. Arthroscopy. 2008;24:113–115. doi: 10.1016/j.arthro.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 85.Lubowitz JH. Anteromedial portal technique for the anterior cruciate ligament femoral socket: pitfalls and solutions. Arthroscopy. 2009;25:95–101. doi: 10.1016/j.arthro.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Rayan F, Nanjayan SK, Quah C, Ramoutar D, Konan S, Haddad FS. Review of evolution of tunnel position in anterior cruciate ligament reconstruction. World J Orthop. 2015;6:252–262. doi: 10.5312/wjo.v6.i2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zantop T, Kubo S, Petersen W, Musahl V, Fu FH. Current techniques in anatomic anterior cruciate ligament reconstruction. Arthroscopy. 2007;23:938–947. doi: 10.1016/j.arthro.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Crawford C, Nyland J, Landes S, Jackson R, Chang HC, Nawab A, Caborn DN. Anatomic double bundle ACL reconstruction: a literature review. Knee Surg Sports Traumatol Arthrosc. 2007;15:946–964; discussion 945. doi: 10.1007/s00167-007-0343-7. [DOI] [PubMed] [Google Scholar]
- 89.Zantop T, Diermann N, Schumacher T, Schanz S, Fu FH, Petersen W. Anatomical and nonanatomical double-bundle anterior cruciate ligament reconstruction: importance of femoral tunnel location on knee kinematics. Am J Sports Med. 2008;36:678–685. doi: 10.1177/0363546508314414. [DOI] [PubMed] [Google Scholar]
- 90.Ho JY, Gardiner A, Shah V, Steiner ME. Equal kinematics between central anatomic single-bundle and double-bundle anterior cruciate ligament reconstructions. Arthroscopy. 2009;25:464–472. doi: 10.1016/j.arthro.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 91.Bedi A, Maak T, Musahl V, O’Loughlin P, Choi D, Citak M, Pearle AD. Effect of tunnel position and graft size in single-bundle anterior cruciate ligament reconstruction: an evaluation of time-zero knee stability. Arthroscopy. 2011;27:1543–1551. doi: 10.1016/j.arthro.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 92.Siebold R. The concept of complete footprint restoration with guidelines for single- and double-bundle ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19:699–706. doi: 10.1007/s00167-010-1376-x. [DOI] [PubMed] [Google Scholar]
- 93.Chhabra A, Kline AJ, Nilles KM, Harner CD. Tunnel expansion after anterior cruciate ligament reconstruction with autogenous hamstrings: a comparison of the medial portal and transtibial techniques. Arthroscopy. 2006;22:1107–1112. doi: 10.1016/j.arthro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 94.Griffith TB, Allen BJ, Levy BA, Stuart MJ, Dahm DL. Outcomes of repeat revision anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:1296–1301. doi: 10.1177/0363546513482568. [DOI] [PubMed] [Google Scholar]
- 95.van Eck CF, Kopf S, Irrgang JJ, Blankevoort L, Bhandari M, Fu FH, Poolman RW. Single-bundle versus double-bundle reconstruction for anterior cruciate ligament rupture: a meta-analysis--does anatomy matter? Arthroscopy. 2012;28:405–424. doi: 10.1016/j.arthro.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 96.Desai N, Björnsson H, Musahl V, Bhandari M, Petzold M, Fu FH, Samuelsson K. Anatomic single- versus double-bundle ACL reconstruction: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2014;22:1009–1023. doi: 10.1007/s00167-013-2811-6. [DOI] [PubMed] [Google Scholar]
- 97.Paxton ES, Kymes SM, Brophy RH. Cost-effectiveness of anterior cruciate ligament reconstruction: a preliminary comparison of single-bundle and double-bundle techniques. Am J Sports Med. 2010;38:2417–2425. doi: 10.1177/0363546510375545. [DOI] [PubMed] [Google Scholar]
- 98.Hofbauer M, Muller B, Murawski CD, Baraga M, van Eck CF, Fu FH. Strategies for revision surgery after primary double-bundle anterior cruciate ligament (ACL) reconstruction. Knee Surg Sports Traumatol Arthrosc. 2013;21:2072–2080. doi: 10.1007/s00167-013-2470-7. [DOI] [PubMed] [Google Scholar]
- 99.Herbort M, Tecklenburg K, Zantop T, Raschke MJ, Hoser C, Schulze M, Petersen W, Fink C. Single-bundle anterior cruciate ligament reconstruction: a biomechanical cadaveric study of a rectangular quadriceps and bone--patellar tendon--bone graft configuration versus a round hamstring graft. Arthroscopy. 2013;29:1981–1990. doi: 10.1016/j.arthro.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 100.Grøntvedt T, Engebretsen L, Benum P, Fasting O, Mølster A, Strand T. A prospective, randomized study of three operations for acute rupture of the anterior cruciate ligament. Five-year follow-up of one hundred and thirty-one patients. J Bone Joint Surg Am. 1996;78:159–168. doi: 10.2106/00004623-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Eggli S, Kohlhof H, Zumstein M, Henle P, Hartel M, Evangelopoulos DS, Bonel H, Kohl S. Dynamic intraligamentary stabilization: novel technique for preserving the ruptured ACL. Knee Surg Sports Traumatol Arthrosc. 2015;23:1215–1221. doi: 10.1007/s00167-014-2949-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kohl S, Evangelopoulos DS, Ahmad SS, Kohlhof H, Herrmann G, Bonel H, Eggli S. A novel technique, dynamic intraligamentary stabilization creates optimal conditions for primary ACL healing: a preliminary biomechanical study. Knee. 2014;21:477–480. doi: 10.1016/j.knee.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 103.Kohl S, Stock A, Ahmad SS, Zumstein M, Keel M. Dynamic intraligamentary stabilization and primary repair: A new concept for the treatment of knee dislocation. Injury. 2015;46:724–728. doi: 10.1016/j.injury.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 104.Heitmann M, Gerau M, Hötzel J, Giannakos A, Frosch KH, Preiss A. [Ligament bracing--augmented primary suture repair in multiligamentous knee injuries] Oper Orthop Traumatol. 2014;26:19–29. doi: 10.1007/s00064-013-0263-2. [DOI] [PubMed] [Google Scholar]


