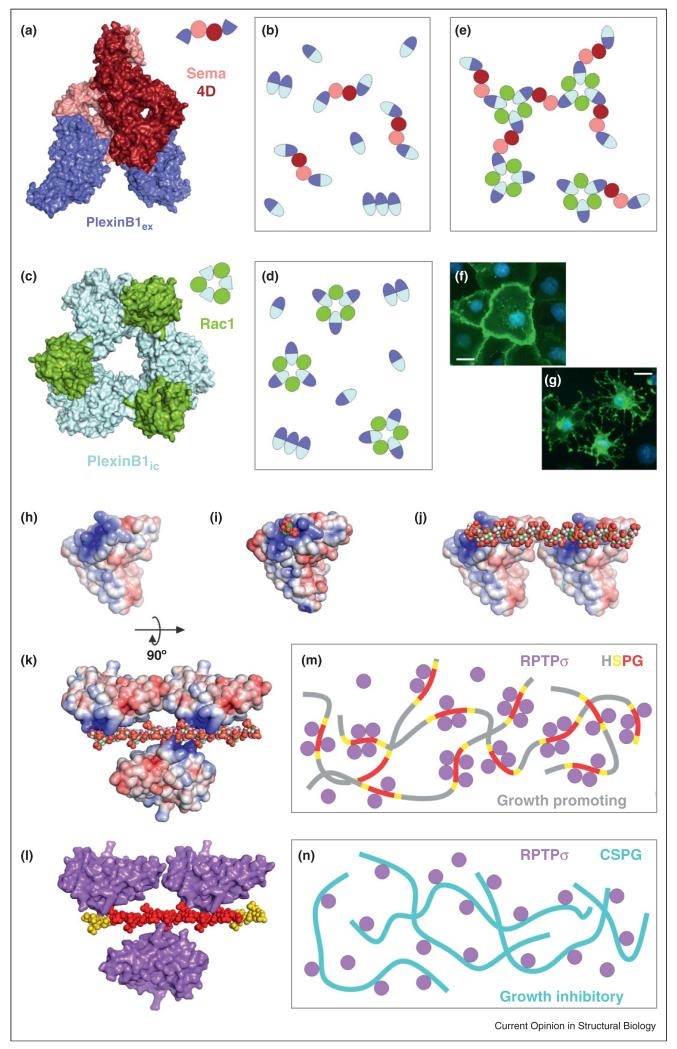
Figure 3.
Emerging concepts in cell surface signaling: examples of combined structure/function approaches that have revealed supramolecular receptor organization. Such assemblies are driven by either protein–protein interactions (e.g. the semaphoring/plexin system) or by non-protein ligands (e.g. type IIa RPTPs interactions with HSPGs and CSPGs). (a) Crystal structure of a Sema4D-PlexinB1 ectodomain complex [31••]. (b) Binding of dimeric Sema4D ligands (pink/red) to the PlexinB1 extracellular region (dark blue) triggers receptor dimerization. Note that non-ligand-dependent PlexinB1 ectodomain oligomerization has also been reported, but its extent or architecture are still unclear [31••]. (c) Crystal structure of the PlexinB1 intracellular region in complex with Rac1 [61•]. (d) This interaction leads to the formation of a 3:3 receptor–ligand assembly. (e) Schematic representation of the putative impact of simultaneous Sema4D and Rac1 binding to PlexinB1. The combined 2-fold and 3-fold interactions may lead to a hexagonal ‘honeycomb’ arrangement (shown in part here) that facilitates bi-directional signaling. (f)–(g) COS7 cell collapse assays were used to validate crystallographic interfaces using structure-guided mutagenesis. Scalebar: 40 μm. (h) Electrostatic potential representation (±5kT/e) of the glycosaminoglycan (GAG)-binding region of human RPTPσ [45••]. (i) A basic residue cluster, conserved in all family members, interacts with sulphated sugars (shown here is human LAR in complex with sucrose-octasulphate). (j)–(k) The polymeric nature of heparan sulphate (HS) triggers receptor clustering. (l)–(m) Importantly, the distribution of sulphate groups along the HS chains is not even: 12–14 sulphate-rich units (red), flanked by intermediate sulphation regions (yellow) are separated by long low-sulphation portions. This imposes an uneven distribution of receptors on the cell surface (~four receptors per cluster), which is essential to promote neuronal motility [45••]. (n) In contrast, the distribution of sulphate groups on chondroitin sulphate molecules prevents formation of RPTPσ clusters and inhibits cell motility [45••].