Abstract
N-3 polyunsaturated fatty acids (PUFA) exert immunomodulatory effects on B cells. We previously demonstrated that n-3 PUFAs enhanced the relative percentage and/or frequency of select B2 cell subsets. The objectives here were to determine if n-3 PUFAs: i) could boost cytokines that target B cell frequency, ii) enhance the frequency of the B1 population and iii) to identify the mechanism by which n-3 PUFAs modify the proportion of B cells. Administration of n-3 PUFAs as fish oil to C57BL/6 mice enhanced secretion of the Th2 cytokine IL-5 but not IL-9 or IL-13. N-3 PUFAs had no influence on the percentage or frequency of peritoneal B1 or B2 cells. Subsequent experiments with IL-5−/− knockout mice showed n-3 PUFAs decreased the percentage of bone marrow B220loIgMhi cells and increased the proportion and number of splenic IgM+IgDloCD21lo cells compared to the control. These results, when compared with our previous findings with wild type mice, suggested IL-5 had no role in mediating the effect of n-3 PUFAs on B cell populations. To confirm this conclusion, we assayed IL-5 secretion in a diet-induced obesity model in which n-3 PUFAs enhanced the frequency of select B cell subsets. N-3 PUFA supplementation as ethyl esters to obesogenic diets did not alter circulating IL-5 levels. Altogether, the data establish that n-3 PUFAs as fish oil can increase circulating IL-5 in lean mice, which has implications for several disease endpoints, but this increase in IL-5 is not the mechanistic link between n-3 PUFAs and changes in B cell populations.
Keywords: EPA, DHA, n-3 PUFAs, B cells, IL-5
Introduction
Long chain n-3 polyunsaturated fatty acids (PUFA) (i.e. omega-3 fats) found in fish oil are bioactive molecules with great potential for manipulating the immune system. Indeed, pre-clinical studies in cell culture and rodent models show that the n-3 PUFAs eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids are immunomodulatory and can influence both innate and adaptive immunity [1, 2]. Supporting clinical studies however have revealed mixed results on the efficacy of n-3 PUFAs for differing immunological outcomes [3-5]. The gap between pre-clinical and clinical studies is likely driven by numerous variables, one of which may be the lack of understanding of the cellular mechanisms by which n-3 PUFAs target the immune system [6]. Once the underlying mechanisms of these fatty acids are better delineated at the pre-clinical level, then more focused clinical studies can be designed.
Recent studies from our laboratory and from others show that n-3 PUFAs can target various aspects of B cell biology in murine models. To exemplify, n-3 PUFAs modify B cell activation, antigen presentation to helper T cells, antibody production, surface expression of select molecules, development in bone marrow, and the relative percentage or frequency of B cells in specific tissues [7-13]. While these studies have established that numerous functional endpoints of B cells are modified with n-3 PUFAs, the underlying mechanisms for the most part remain unknown. In the present study, we tackled one potential underlying mechanism by which n-3 PUFAs could modify the fraction of differing B cell subsets.
B cell growth and development are tightly regulated in response to cytokines secreted from other cell types. In particular, IL-5 is a Th2 cytokine that stimulates proliferation and differentiation of B cells [14, 15]. There is a considerable gap in knowledge on whether n-3 PUFAs can influence the circulating levels of cytokines such as IL-5. Therefore, our first objective was to determine if n-3 PUFAs could enhance IL-5 secretion, in addition to other cytokines, in response to intraperitoneal stimulation with a T-independent antigen. We then determined if n-3 PUFAs influenced the proportion of peritoneal B cells, particularly the IL-5 responsive B1 subset in wild type mice [16]. This was critical to address given that select B1 cells have a beneficial role in metabolic diseases such as atherosclerosis and insulin resistance [17, 18]. Finally, we utilized IL-5 knockout mice to determine if n-3 PUFAs could enhance select B cell subsets in the absence of IL-5 secretion.
Materials and Methods
Mice and diets
Male C57BL/6 or IL5−/− (C57BL/6-Il5tm1Kopf/J) mice, purchased from Jackson Laboratories, consumed a purified control low fat soybean oil enriched diet or a menhaden fish oil enriched diet (containing n-3 PUFAs) for 4 weeks, as previously described [12]. The n-3 PUFA enriched diet contained 1.3% of the total kcal from docosahexaenoic acid (DHA) and 2% from eicosapentaenoic acid (EPA). For a select study, C57BL/6 mice were fed high fat diets supplemented with EPA or DHA ethyl esters, as previously described [13]. Mice were sacrificed via CO2 inhalation followed by cervical dislocation. All of the experiments with mice fulfilled the guidelines established by the East Carolina University Brody School of Medicine for euthanasia and humane treatment.
In vivo injections and serum collection
At week 3 of dietary intervention, the lean mice were intraperitoneal (i.p.) injected with 1 μg of trinitrophenylated-lipopolysaccharide (TNP-LPS, Biosearch Technologies). The rationale for using a T-independent antigen was to remain consistent with our previous work [12]. Seven days following TNP-LPS injection, blood was collected from the superficial temporal vein into a capiject tube (Fisher). The blood was centrifuged for 15 minutes at room temperature at 1300 rpm to separate the serum. The serum was then frozen for cytokine analyses.
Cytokine levels
Cytokines in serum were measured with a multiplex cytokine kit (Milliplex MAP Kit, Millipore, MCYTOMAG-70K) per the manufacturer’s directions. The multiplex assay was analyzed with a Luminex-100, Bio-Plex machine (Bio-Rad) with DD gate adjusted to detect magnetic beads. The Bio-Plex was run with Bio-Plex manager 4.1 software. Serum levels of IL-5 were confirmed with an Elisa MAX Standard (Biolegend), as per manufacturer’s protocol [13].
Analysis of bone marrow B cells
Bone marrow was obtained from the mouse tibia and femur as previously demonstrated [12]. Briefly, each degloved leg was cleaned with a razor to remove all muscle tissue. The bone marrow was flushed with 10 mL RPMI 1640 1× media (Mediatech) supplemented with 5% heat-inactivated defined fetal bovine serum (FBS) (Hyclone), 2 mM L-glutamine, and 1% penicillin/streptomycin and placed into 20 mL of the same media. Once the bone marrow was filtered and the red blood cells lysed, 0.5 × 106 cells were transferred to each well of a 96 well plate. The bone marrow was stained for 10 minutes on ice with FcR Block (Miltenyi Biotec) followed by 20 minutes with an antibody cocktail containing B220-FITC (Miltenyi Biotec), CD19-PerCP/Cy5.5 (Biolegend), IgM-PE (Southern Biotech), IgD-APC (Biolegend) and CD21-APC/Cy7 (Biolegend) in 1× PBS supplemented with 0.1% BSA. Dead cells were stained with Sytox Blue (Invitrogen).
Analysis of splenic B cells
Spleens were mechanically disrupted and red blood cells were osmotically lysed to generate a single cell suspension [13]. B220+ B cells were purified from splenocytes with a negative selection microbead kit obtained from Miltenyi Biotec. 0.5 × 106 splenic B cells were transferred to a 96 well plate and stained for 20 minutes on ice with a combination of B220-FITC (Miltenyi Biotec), IgM-PE (Southern Biotech), IgD-APC (Biolegend), CD19-PerCP/Cy5.5 (Biolegend), CD40-PerCP/Cy5.5 (Biolegend), CD21-APC/Cy7 (Biolegend), and MHC class II-PE (Bio X Cell) in 1× PBS supplemented with 0.1% BSA. The splenic B cell subsets analyzed were IgM+IgDloCD21lo, IgM+IgDhiCD21lo, IgM+IgDhiCD21hi, and IgM+IgDloCD21hi [12, 19].
Analysis of peritoneal B cells
The outer skin of the mouse was removed from the peritoneum, exposing the inner skin lining [20]. 5 ml of cold 1× PBS supplemented with 3% FBS was injected into the peritoneal cavity with a 27G needle and then the peritoneal cavity was gently massaged. A small incision was made in the inner skin and a disposable pipette was utilized to collect the 1× PBS supplemented with 3% FBS. 0.5 × 106 cells were transferred to a 96 well plate and stained for 20 minutes on ice with a combination of CD19-PerCP/Cy5.5 (Biolegend) and CD23-PE/Cy7 (Biolegend) in 1× PBS supplemented with 0.1% BSA. The peritoneal subsets analyzed were CD19hiCD23lo (B1) and CD19hiCD23hi (B2) [20].
Flow cytometry
B cells were stained with fluorophore labeled antibodies as described above [12]. Data were acquired with a BD LSR II flow cytometer. Analysis of the B cell subsets were based on the percentage of live cells and gating strategies are presented throughout the results.
Statistical analysis
For the majority of the studies, the data are from 4-8 independent experiments (with one mouse per diet used per experiment) as indicated in the figure legends. The data sets showed normalized distributions and were analyzed using GraphPad Prism. Statistical significance was established using an unpaired two-tailed t test or a one-way ANOVA followed by a post-hoc Bonferroni multiple comparison t test. P values less than 0.05 were considered to be significant.
Results
N-3 PUFAs enhanced circulating levels of IL-5 in C57BL/6 mice but had no influence on the percentage of B1 cells in the peritoneal cavity
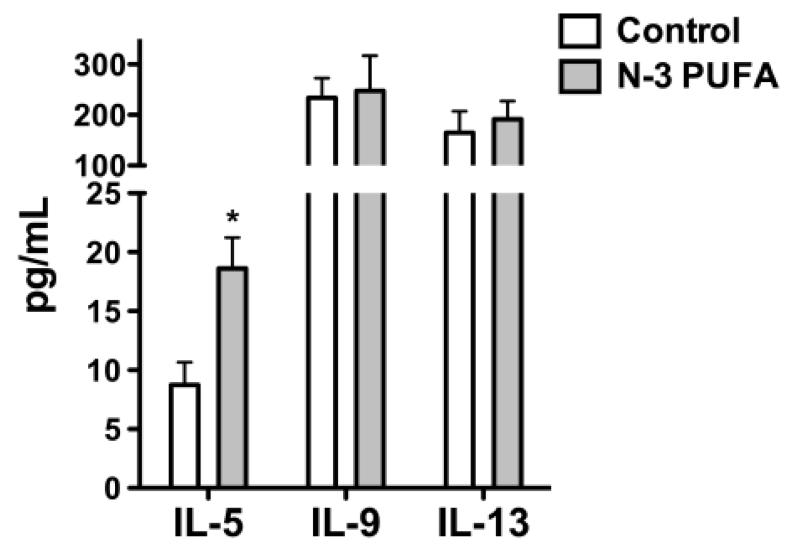
We first determined if n-3 PUFAs enhanced the levels of select circulating cytokines known to influence B cell proliferation, differentiation, and/or development. Analysis of cytokine secretion in response to i.p. TNP-LPS stimulation revealed that n-3 PUFAs increased IL-5 secretion by 2.1 fold compared to the control (Figure 1). N-3 PUFAs had no effect on either IL-9 or IL-13 levels (Figure 1).
Figure 1. N-3 PUFAs enhance serum IL-5 levels in C57BL/6 mice.
Serum levels of IL-5, IL-9, and IL-13 from mice fed n-3 PUFAs for 4 weeks. Mice were injected with 1μ of TNP-LPS on week 3 and serum was isolated 7 days post-injection. Values are average ± S.E. from 5-6 independent experiments. Asterisk indicates statistical significance relative to the control diet: *p<0.05.
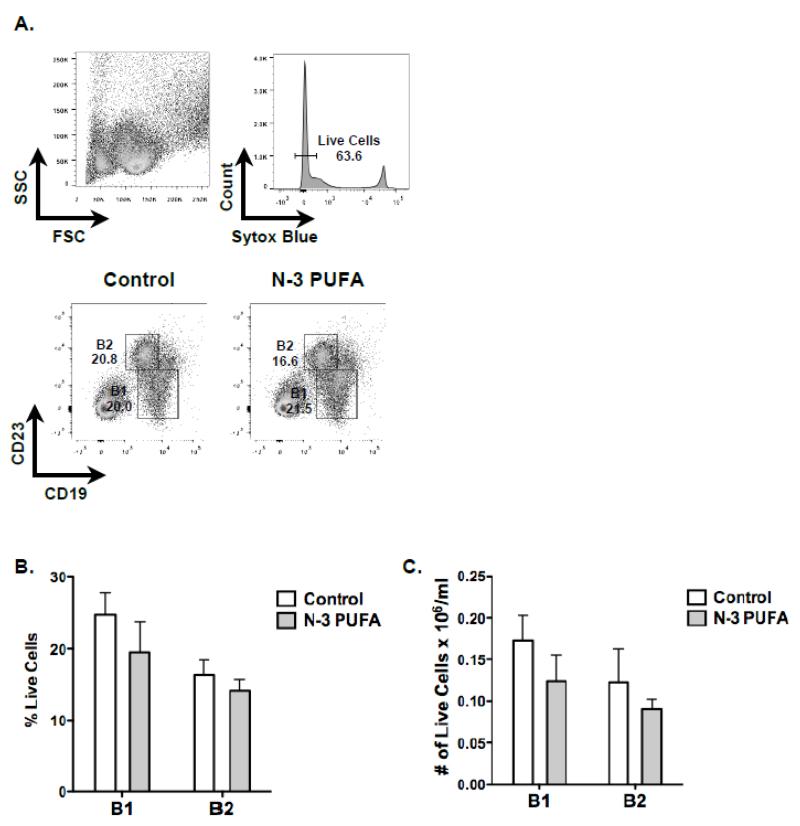
We next tested the hypothesis that increased IL-5 secretion in wild type mice would increase the proportion of B1 cells in the peritoneal cavity. The flow cytometry gating strategy for peritoneal B1 and B2 cells is depicted in Figure 2A. As expected, the percentage of B1 cells (~20-25%) was generally higher in the peritoneal cavity than B2 cells (~15%) (Figure 2B). However, there was no influence of n-3 PUFAs on the relative percentage of either B1 or B2 subsets. Similarly, the frequency of B1 and B2 cells was not modified by n-3 PUFAs (Figure 2C).
Figure 2. N-3 PUFAs have no influence on the percentage of B1 and B2 cells in the peritoneal cavity of wild type mice.
(A) Flow cytometry gating strategy for analyzing B1 and B2 cells in the peritoneal cavity. (B) Percentage and (C) frequency of B1 and B2 cell subsets. Mice were injected with 1μg of TNP-LPS on week 3 and analyses were conducted one week later. Values are average ± S.E. from 4 independent experiments.
N-3 PUFAs decreased the percentage of bone marrow B220loIgMhi cells in IL-5−/− mice
We have previously shown that n-3 PUFAs modify the proportion of select B cell subsets in the bone marrow and spleen [12]. Given that IL-5 has a role in influencing these populations, we pursued studies in an IL-5 knockout model (IL-5−/−) to determine if increased IL-5 in response to n-3 PUFAs is mechanistically driving the changes in select populations. We first confirmed that IL-5 levels were low in IL-5−/− mice that were fed control or n-3 PUFA diets. Cytokine analysis showed that IL-5 levels were essentially not detectable with IL-5−/− mice consuming either the control or n-3 PUFA enriched diet (Supplemental Figure 1).
The gating strategy for the bone marrow cells was the same as previously shown [12]. Analysis of the relative proportion of bone marrow cells showed that n-3 PUFAs had no effect on the B220hiIgMlo cells and had a tendency to decrease the B220hiIgMhi population compared to the control (Supplemental Figure 2). The bone marrow B220loIgMhi population was decreased by approximately a third with mice fed the n-3 PUFA diet relative to mice consuming the control diet.
N-3 PUFAs manipulated the percentage and frequency of select splenic B cells in IL-5−/− mice
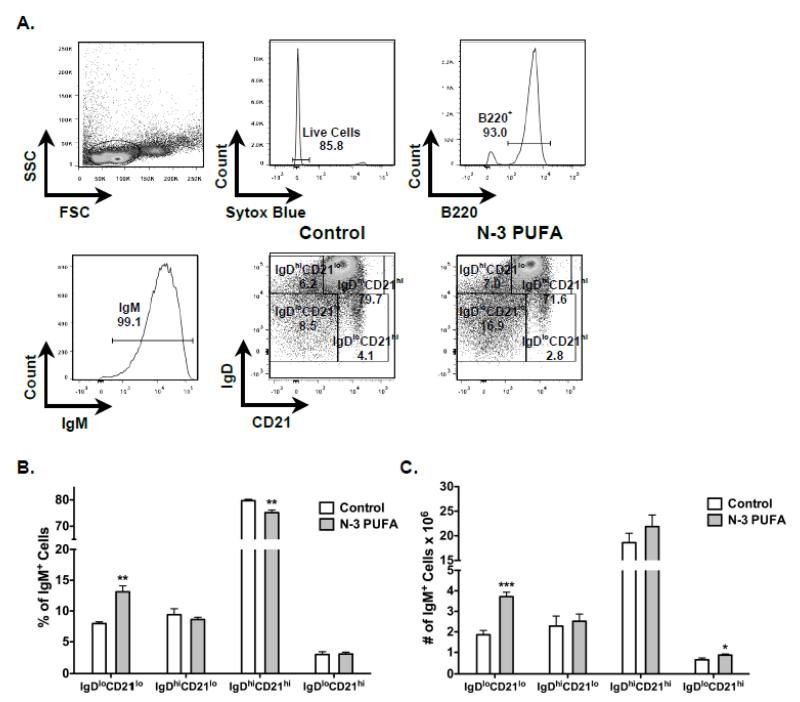
We also measured the effect of n-3 PUFAs on splenic B cell subsets in IL-5−/− mice using flow cytometry (Figure 3A). Relative to the control, n-3 PUFAs increased the percentage of IgM+IgDloCD21lo cells by 1.6 fold whereas the percentage of IgM+IgDhiCD21hi cells was minimally lowered by ~5% (Figure 3B). N-3 PUFAs had no effect on the percentage of IgM+IgDhiCD21lo and IgM+IgDloCD21hi cells (Figure 3B).
Figure 3. N-3 PUFAs modify the percentage and frequency of splenic IgM+IgDloCD21lo and IgM+IgDhiCD21hi B cells in IL-5−/− mice.
(A) Sample gating strategy for analysis for differing B cell subsets. (B) Percentage and (C) frequency of differing IgM+ B cell subsets from control and n-3 PUFA fed mice. Values are average ± S.E. from 5-6 independent experiments. Asterisks indicate statistical significance relative to the control diet: *p<0.05, **p<0.01, ***p<0.001.
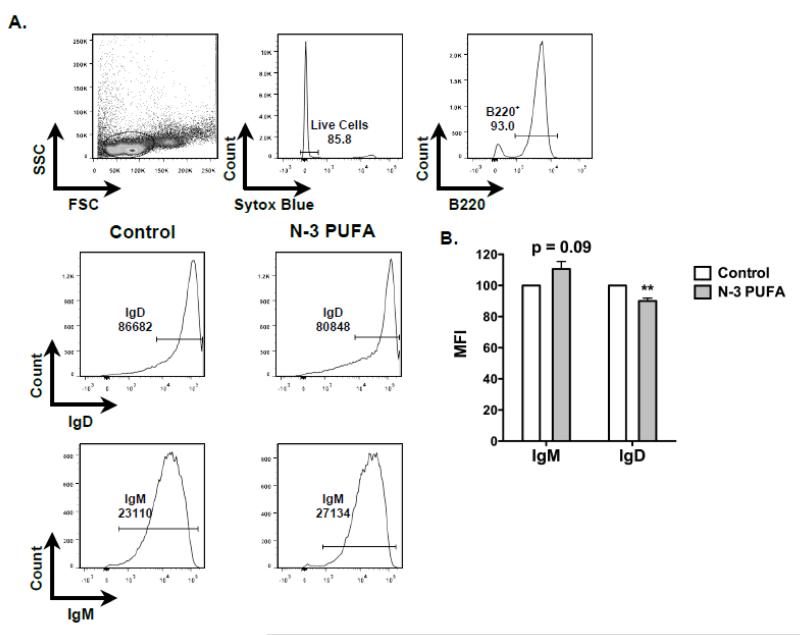
We calculated the frequency of B cells upon n-3 PUFA intervention. The number of B cells on average in the control mice was 25.9 × 106 cell per spleen compared to 32 × 106 cells per spleen for mice consuming n-3 PUFAs. The increase in the number of B cells with n-3 PUFAs showed a trend but failed to reach statistical significance. Nevertheless, there were twice as many IgM+IgDloCD21lo cells in the spleens of mice consuming n-3 PUFAs compared mice fed the control (Figure 3C). The number of IgM+IgDloCD21hi cells increased modestly by 1.3 fold with n-3 PUFAs. The frequency of IgM+IgDhiCD21lo and IgM+IgDhiCD21hi cells was not affected by n-3 PUFAs. We also measured the surface expression of splenic B cell IgM and IgD with flow cytometry (Figure 4A). Analysis of mean fluorescence intensities showed a trend for cell surface IgM expression to be about 10% higher on the splenic B cells from mice fed n-3 PUFAs compared to splenic B cells from control mice (p =0.09). There was a 10% decrease in cell surface IgD expression on splenic B cells from mice fed n-3 PUFAs relative to the control diet (Figure 4B).
Figure 4. N-3 PUFAs lower splenic B cell surface IgD expression of IL-5−/− mice.
(A) Flow cytometry gating strategy for analysis of surface IgM and IgD expression. (B) Normalized mean intensity of surface IgM and IgD of B cells. Fluorescence intensity values required normalization since experimental settings were adjusted between experiments. Values are average ± S.E. from 5-6 independent experiments. Asterisks indicate statistical significance relative to the control diet: **p<0.01.
IL-5 secretion is not enhanced with n-3 PUFAs in C57BL/6 obese mice
The increase in the frequency of select B cell subsets (Figure 3) after n-3 PUFA feeding in IL-5−/− mice suggested that increased IL-5 is not the mechanism by which n-3 PUFAs affect these populations. To confirm this, we measured circulating IL-5 levels in a diet-induced obesity model using C57BL/6 mice in which we had previously demonstrated that n-3 PUFAs enhanced the percentage and/or frequency of the IgM+IgDloCD21lo subset [13]. Administration of EPA or DHA ethyl esters as part of a high fat diet had no influence on serum IL-5 levels (Supplemental Figure 3).
Discussion
This study significantly advances the field of n-3 PUFAs and immunity by answering the following questions: i) do n-3 PUFAs increase the secretion of select Th2 cytokines, particularly IL-5? ii) how are the B1 and B2 repertoires in the peritoneal cavity influenced by dietary supplementation with n-3 PUFAs in C57BL/6 mice? iii) are B cell subsets increased with dietary n-3 PUFA consumption in IL-5−/− mice? Data from these studies reveal that n-3 PUFAs enhance IL-5 secretion but this enhanced IL-5 production does not mechanistically drive the reported changes in select B cell populations, as these changes generally continued to be observed in the absence of IL-5 production.
The potential role of n-3 PUFAs in boosting Th2 cytokines
Th2 cytokines regulate various aspects of B cell biology and vice versa [21, 22]. IL-5, in particular, stimulates proliferation and differentiation of B cells and eosinophils [15]. IL-5 is also important for the induction of IgA class switching in mucosal B cells, protection against parasitic infection, and pathology of asthma due to stimulation of eosinophils [15]. In addition, B-1 cells constitutively express the IL-5 receptor and use IL-5 for survival, proliferation and differentiation to natural antibody-secreting plasma cells [14]. Therefore, IL-5 has a critical role in immunity and it was essential to understand how n-3 PUFAs influence IL-5 secretion in vivo.
Our data demonstrate that n-3 PUFAs in menhaden fish oil increased the levels of IL-5 upon i.p. TNP-LPS stimulation. The increased IL-5 secretion is consistent with Gurzell et al., who demonstrated that administration of DHA-enriched fish oil to Smad3−/− mice enhanced the frequency of B cells and increased secretion of IL-5, IL-9, and IL-13 in circulation [10]. Schuster et al. also demonstrated in an airway inflammation model that n-3 PUFAs had a tendency to increase IL-5 secretion [23]. It is important to note that IL-5 is implicated in the pathogenesis of several diseases, particularly lung inflammatory conditions such as asthma [24]. The findings from this study and others imply that an enhancement in IL-5 secretion in humans could have negative consequences for inflammatory lung diseases. However, at this stage it is premature to assume that increased IL-5 levels will lead to exacerbated disease. In fact, n-3 PUFAs may have a beneficial role in preventing lung inflammation in asthma and there is even evidence to show that n-3 PUFAs can lower IL-5 levels [25, 26].
At the cellular level, there is some suggestion that n-3 PUFAs can alter Th2 cytokines. For instance, n-3 PUFAs in a mouse model increased the secretion of IL-4 by targeting the function of a mixture of antigen presenting cells [27]. On the other hand, n-3 PUFAs suppressed the production of Th1 cytokines upon stimulation with anti-CD3/CD28 antibodies or hybridomas with no influence on Th2 cytokines [28, 29]. Jang get al., illustrated that fat-1 mice, which constitutively produce n-3 PUFAs in the absence of dietary intervention, decreased Th2 cytokines and moreover there was a decrease in lung infiltration of select inflammatory cell types [30].
IL-5 is not driving changes in the percentage and/or frequency of B1 or IgM+IgDloCD21lo cells
A recent manuscript showed that n-3 PUFAs enhanced the number of peritoneal B1 cells upon immunization with methylated BSA [31]. Given that B1 cells express the IL-5 receptor and are highly sensitive to IL-5 levels, we determined if n-3 PUFAs enhanced the frequency of B1 cells upon TNP-LPS stimulation. Our assays revealed no influence of n-3 PUFAs on B1 cells. We even conducted a few select studies measuring B1a B cells and there was no effect of n-3 PUFAs on this specific subset (data not shown).
We previously reported that n-3 PUFAs enhanced the percentage and frequency of IgM+IgDloCD21lo cells upon intervention with TNP-LPS stimulation [12]. Furthermore, we also observed an increase in the frequency of this population upon supplementing high fat diets with menhaden fish oil or EPA or DHA ethyl esters, which has implications for humoral immunity in obesity [12, 13, 19]. The IgM+IgDloCD21lo population of B cells represents transitional 1 and B1 cells, which are likely the most receptive to an increase in IL-5 levels in the spleen. Therefore, if IL-5 was the mechanistic link to drive the enhancement in this population with n-3 PUFAs, then we would have expected the number of cells within this population to have remain unchanged between the control and n-3 PUFA diets with IL-5 knockout mice.
We acknowledge that there were some differences between our previous work with wild type mice and the results observed here with IL-5−/− animals in response to n-3 PUFAs [12]. Namely, the number of total B cells was not elevated and an increase in surface IgM levels failed to reach significance with n-3 PUFAs; nevertheless, there was a trend for enhanced membrane IgM expression and increased splenic B cells in the knockout model. Finally, it is important to note that we verified the conclusion that IL-5 was not driving an increase in the IgM+IgDloCD21lo population by assaying for IL-5 levels in response to administration of EPA or DHA ethyl esters supplemented in high fat diets.
There are some limitations of the study. Namely, we did not assay for IgM and IgD expression in the bone marrow with the IL-5 knockout mice. We have assayed for IgM expression in bone marrow with mice on a 129 background and have not found an effect (unpublished results). Our future studies will need to address how n-3 PUFAs influence development of B cells in the bone marrow. There is a recent study to suggest that high doses of fish oil diminish the development of mature B cells in the bone marrow in addition to influencing the development of other cell types such as neutrophils [11, 32]. Furthermore, we will need to determine how B cell class switching and formation of germinal centers may be modified in response to EPA and DHA. Finally, we did not test for IgA expression in the bone marrow or with splenic cells. This will be essential to determine, particularly in light of our previous data to show that fecal IgA is elevated [10, 13].
Other potential mechanisms of n-3 PUFAs on B cells
It is important to consider what other mechanisms may be driving the production of select B cell subsets, which is an area of future investigation. One likely possibility is that dietary n-3 PUFAs, which serve as substrates for specific enzymes, are enhancing the production of pro-resolving lipid mediators [33]. Indeed, there is evidence in the literature to show that dietary n-3 PUFA supplementation can increase levels of pro-resolving lipid mediators, although there are some labs that question this possibility [34-36]. Furthermore, Phipps and co-workers have demonstrated that 17-HDHA, a hydroxylated DHA molecule, enhances the production of antibody secreting cells in vitro and in vivo [37, 38]. Therefore, we speculate that metabolo-lipidomics analyses will shed light on how n-3 PUFAs as dietary supplements may be influencing the concentrations of differing specialized pro-resolving lipid mediators and thereby the frequency of B cell subsets. Other potential mechanisms of n-3 PUFAs include the possibility that n-3 PUFAs are targeting B cell development and promoting trafficking in circulation to select tissues. At a molecular level, n-3 PUFAs could be driving changes in B cell lipid microdomain organization, signaling and gene expression [39, 40].
Conclusion
In summary, our data establish that short-term intake of n-3 PUFAs can increase the circulating levels of IL-5 with no influence on the percentage or frequency of B1 cells in C57BL/6 mice. Furthermore, using IL-5−/− mice we demonstrate that n-3 PUFAs increase the numbers of select B cell subsets in the absence of IL-5 production. Therefore, we have ruled out n-3 PUFA driven IL-5 production as a mechanism by which n-3 PUFAs manipulate splenic B cell populations.
Supplementary Material
Supplemental Figure 1: IL-5 secretion is not detectable in IL-5−/− mice. Serum levels of IL-5 from IL-5−/− mice. Cytokine levels were at the lower detection limit. Data are average ± S.E. from 2 independent experiments.
Supplemental Figure 2: N-3 PUFAs decrease the percentage of B220loIgMhi cells in the bone marrow of IL-5−/− mice. Percentage of B220hiIgMlo, B220loIgMhi, and B220hiIgMhi cells in response to administering control and n-3 PUFA enriched diets for 4 weeks. Mice were injected with 1μg of TNP-LPS on week 3 and analyses were conducted one week later. Values are average ± S.E. from 5-6 independent experiments. Asterisk indicates statistical significance relative to the control diet: *p<0.05.
Supplemental Figure 3: IL-5 secretion is not enhanced with n-3 PUFAs upon administration of high fat diets to wild type mice. IL-5 levels for C57BL/6 mice consuming high fat diets supplemented with either EPA or DHA ethyl esters. Mice were fed for 10 weeks followed by isolation of serum. Values are average ± S.E. from 7-8 independent experiments.
Acknowledgements
We thank Rasagna Kosaraju for her assistance with the IL-5 knockout studies. This work was supported in part by a grant from the National Center for Complementary and Integrative Health at the NIH (R01AT008375) to S.R.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest and all authors have approved the manuscript.
References
- [1].Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 2015;1851:469–84. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- [2].Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergology International. 2015;64:27–34. doi: 10.1016/j.alit.2014.08.003. [DOI] [PubMed] [Google Scholar]
- [3].Skulas-Ray AC. Omega-3 fatty acids and inflammation: A perspective on the challenges of evaluating efficacy in clinical research. Prostaglandins & Other Lipid Mediators. 2015;116-117C:104–11. doi: 10.1016/j.prostaglandins.2015.02.001. [DOI] [PubMed] [Google Scholar]
- [4].Miles EA, Calder PC. Influence of marine n-3 polyunsaturated fatty acids on immune function and a systematic review of their effects on clinical outcomes in rheumatoid arthritis. The British Journal of Nutrition. 2012;107(Suppl 2):S171–84. doi: 10.1017/S0007114512001560. [DOI] [PubMed] [Google Scholar]
- [5].Nigam A, Talajic M, Roy D, Nattel S, Lambert J, Nozza A, et al. Fish oil for the reduction of atrial fibrillation recurrence, inflammation, and oxidative stress. Journal of the American College of Cardiology. 2014;64:1441–8. doi: 10.1016/j.jacc.2014.07.956. [DOI] [PubMed] [Google Scholar]
- [6].Calder PC. Mechanisms of action of (n-3) fatty acids. The Journal of Nutrition. 2012;142:592S–9S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- [7].Rockett BD, Salameh M, Carraway K, Morrison K, Shaikh SR. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. Journal of Lipid Research. 2010;51:1284–97. doi: 10.1194/jlr.M000851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rockett BD, Teague H, Harris M, Melton M, Williams J, Wassall SR, et al. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. Journal of Lipid Research. 2012;53:674–85. doi: 10.1194/jlr.M021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rockett BD, Melton M, Harris M, Bridges LC, Shaikh SR. Fish oil disrupts MHC class II lateral organization on the B-cell side of the immunological synapse independent of B-T cell adhesion. The Journal of Nutritional Biochemistry. 2013;24:1810–6. doi: 10.1016/j.jnutbio.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gurzell EA, Teague H, Harris M, Clinthorne J, Shaikh SR, Fenton JI. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. Journal of Leukocyte Biology. 2012;93:463–470. doi: 10.1189/jlb.0812394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gurzell EA, Teague H, Duriancik D, Clinthorne J, Harris M, Shaikh SR, et al. Marine fish oils are not equivalent with respect to B-cell membrane organization and activation. The Journal of Nutritional Biochemistry. 2015;26:369–77. doi: 10.1016/j.jnutbio.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Teague H, Fhaner CJ, Harris M, Duriancik DM, Reid GE, Shaikh SR. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. Journal of Lipid Research. 2013;54:3130–8. doi: 10.1194/jlr.M042457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Teague H, Harris M, Fenton J, Lallemand P, Shewchuk B, Shaikh SR. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. Journal of Lipid Research. 2014;55:1420–1433. doi: 10.1194/jlr.M049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Takatsu K, Kouro T, Nagai Y. Chapter 6 Interleukin 5 in the Link Between the Innate and Acquired Immune Response. In: Frederick WA, editor. Advances in Immunology. Academic Press; 2009. pp. 191–236. [DOI] [PubMed] [Google Scholar]
- [15].Takatsu K. Interleukin 5 and B cell differentiation. Cytokine & Growth Factor Reviews. 1998;9:25–35. doi: 10.1016/s1359-6101(97)00034-8. [DOI] [PubMed] [Google Scholar]
- [16].Moon B-g, Takaki S, Miyake K, Takatsu K. The Role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. The Journal of Immunology. 2004;172:6020–9. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- [17].Tsiantoulas D, Diehl CJ, Witztum JL, Binder CJ. B Cells and humoral immunity in atherosclerosis. Circulation Research. 2014;114:1743–56. doi: 10.1161/CIRCRESAHA.113.301145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shen L, Yen Chng MH, Alonso MN, Yuan R, Winer DA, Engleman EG. B-1a lymphocytes attenuate insulin resistance. Diabetes. 2014;64:593–603. doi: 10.2337/db14-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shaikh SR, Haas KM, Beck MA, Teague H. The effects of diet-induced obesity on B cell function. Clinical & Experimental Immunology. 2015;179:90–99. doi: 10.1111/cei.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ray A, Dittel BN. Isolation of mouse peritoneal cavity cells. Journal of Visualized Experiments : JoVE. 2010;35 doi: 10.3791/1488. doi 10.3791/1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].León B, Ballesteros-Tato A, Lund FE. Dendritic cells and B cells: Unexpected partners in Th2 development. The Journal of Immunology. 2014;193:1531–7. doi: 10.4049/jimmunol.1400149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vazquez MI, Catalan-Dibene J, Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine. doi: 10.1016/j.cyto.2015.02.007. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schuster GU, Bratt JM, Jiang X, Pedersen TL, Grapov D, Adkins Y, et al. Dietary long-chain omega-3 fatty acids do not diminish eosinophilic pulmonary inflammation in mice. American Journal of Respiratory Cell and Molecular Biology. 2014;50:626–636. doi: 10.1165/rcmb.2013-0136OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vercelli D, Gozdz J, von Mutius E. Innate lymphoid cells in asthma: when innate immunity comes in a Th2 flavor. Current Opinion in Allergy and Clinical Immunology. 2014;14:29–34. doi: 10.1097/ACI.0000000000000023. [DOI] [PubMed] [Google Scholar]
- [25].Bargut TC, Ferreira TP, Daleprane JB, Martins MA, Silva PM, Aguila MB. Fish oil has beneficial effects on allergen-induced airway inflammation and hyperreactivity in mice. PloS one. 2013;8:e75059. doi: 10.1371/journal.pone.0075059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bilal S, Haworth O, Wu L, Weylandt KH, Levy BD, Kang JX. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochimica et Biophysica Acta. 2011;1812:1164–9. doi: 10.1016/j.bbadis.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Petursdottir DH, Hardardottir I. Dietary fish oil decreases secretion of T helper (Th) 1-type cytokines by a direct effect on murine splenic T cells but enhances secretion of a Th2-type cytokine by an effect on accessory cells. The British Journal of Nutrition. 2009;101:1040–6. doi: 10.1017/S0007114508048290. [DOI] [PubMed] [Google Scholar]
- [28].Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. The Journal of Nutrition. 2005;135:1745–51. doi: 10.1093/jn/135.7.1745. [DOI] [PubMed] [Google Scholar]
- [29].Kim W, Fan YY, Barhoumi R, Smith R, McMurray DN, Chapkin RS. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. The Journal of Immunology. 2008;181:6236–43. doi: 10.4049/jimmunol.181.9.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Jang H-Y, Lim K, Lee S-M, Park B-H. Effects of n-3 PUFA on the CD4+ type 2 helper T-cell-mediated immune responses in Fat-1 mice. Molecular Nutrition & Food Research. 2014;58:365–75. doi: 10.1002/mnfr.201300194. [DOI] [PubMed] [Google Scholar]
- [31].Tomasdottir V, Thorleifsdottir S, Vikingsson A, Hardardottir I, Freysdottir J. Dietary omega-3 fatty acids enhance the B1 but not the B2 cell immune response in mice with antigen-induced peritonitis. The Journal of Nutritional Biochemistry. 2014;25:111–7. doi: 10.1016/j.jnutbio.2013.09.010. [DOI] [PubMed] [Google Scholar]
- [32].Duriancik DM, Comstock SS, Langohr IM, Fenton JI. High levels of fish oil enhance neutrophil development and activation and influence colon mucus barrier function in a genetically susceptible mouse model. The Journal of Nutritional Biochemistry. doi: 10.1016/j.jnutbio.2015.06.002. In press. [DOI] [PubMed] [Google Scholar]
- [33].Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. American Journal of Physiology - Cell Physiology. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Neuhofer A, Zeyda M, Mascher D, Itariu BK, Murano I, Leitner L, et al. Impaired local production of proresolving lipid mediators in obesity and 17-HDHA as a potential treatment for obesity-associated inflammation. Diabetes. 2013;62:1945–56. doi: 10.2337/db12-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Skarke C, Alamuddin N, Lawson JA, Ferguson JF, Reilly MP, FitzGerald GA. Bioactive products formed in humans from fish oils. Journal of lipid research. 2015 doi: 10.1194/jlr.M060392. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ramon S, Gao F, Serhan CN, Phipps RP. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. The Journal of Immunology. 2012;189:1036–42. doi: 10.4049/jimmunol.1103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, et al. The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: A new class of adjuvant? The Journal of Immunology. 2014;193:6031–40. doi: 10.4049/jimmunol.1302795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shaikh SR, Jolly CA, Chapkin RS. n-3 Polyunsaturated fatty acids exert immunomodulatory effects on lymphocytes by targeting plasma membrane molecular organization. Molecular Aspects of Medicine. 2012;33:46–54. doi: 10.1016/j.mam.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Calder PC. Editorial: Fat chance to enhance B cell function. Journal of Leukocyte Biology. 2013;93:457–9. doi: 10.1189/jlb.1212646. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: IL-5 secretion is not detectable in IL-5−/− mice. Serum levels of IL-5 from IL-5−/− mice. Cytokine levels were at the lower detection limit. Data are average ± S.E. from 2 independent experiments.
Supplemental Figure 2: N-3 PUFAs decrease the percentage of B220loIgMhi cells in the bone marrow of IL-5−/− mice. Percentage of B220hiIgMlo, B220loIgMhi, and B220hiIgMhi cells in response to administering control and n-3 PUFA enriched diets for 4 weeks. Mice were injected with 1μg of TNP-LPS on week 3 and analyses were conducted one week later. Values are average ± S.E. from 5-6 independent experiments. Asterisk indicates statistical significance relative to the control diet: *p<0.05.
Supplemental Figure 3: IL-5 secretion is not enhanced with n-3 PUFAs upon administration of high fat diets to wild type mice. IL-5 levels for C57BL/6 mice consuming high fat diets supplemented with either EPA or DHA ethyl esters. Mice were fed for 10 weeks followed by isolation of serum. Values are average ± S.E. from 7-8 independent experiments.