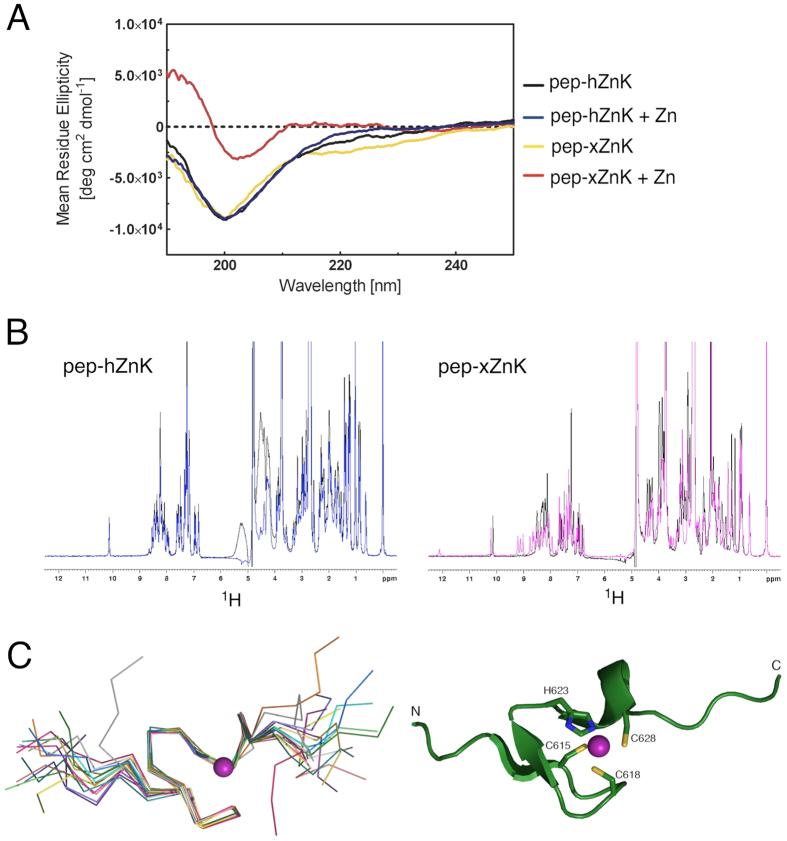
Figure 2. Spectroscopic studies of the peptides corresponding to the human and Xenopus Zn knuckles (pep-hZnK and pep-xZnK).
(A) The CD spectra of the two peptides are characteristic of unfolded proteins (black and yellow, respectively). The addition of Zn2+ in equimolar amount, triggers the formation of secondary structures for the Xenopus peptide but not for the human one. (B) Left: 1D 1H NMR spectra of pep-hZnK in absence (black) and presence (blue) of Zn2+. Right: 1D 1H NMR spectra of pep-xZnK in absence (black) and presence (pink) of Zn2+. (C) Left: NMR bundle of the best 15 structures for the Xenopus Zn knuckle peptide (pep-xZnK). Right: Cartoon representation of one representative NMR structure. The Zn2+ metal ion is in purple and residues involved in Zn2+ coordination are depicted as ball-and-sticks.