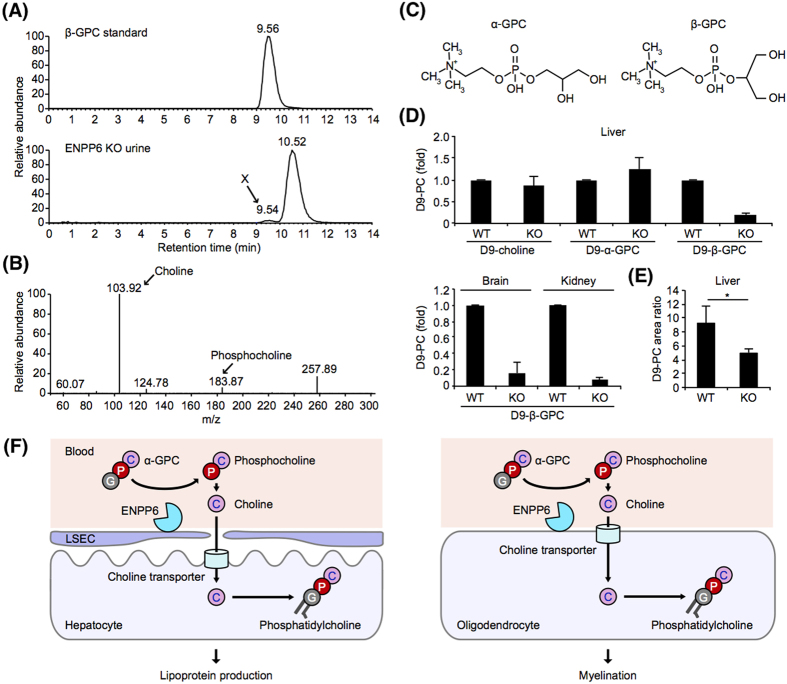
Figure 4. Identification of β-GPC and its metabolism in ENPP6 KO mice.
(A) Identification of β-GPC in the urine of ENPP6 KO mice. Mass chromatogram of chemically synthesized β-GPC (monitored ion m/z 257.9) on normal-phase column chromatography (upper). Mass chromatograms of α-GPC (retention time 10.52 min) and compound X (retention time 9.54 min) detected in urine of ENPP6 KO mice (monitored ion m/z 257.9) showed that compound X has an identical retention times with β-GPC (lower). (B) Mass spectrum of compound X, showing compound X has choline and phosphocholine residues in its structure. (C) Structures of α- and β-GPC. (D,E) Incorporation of D-choline, D-α-GPC and D-β-GPC to phosphatidylcholine in various tissues. D-choline, D-α-GPC or D-β-GPC was injected into wild-type and ENPP6 KO mice via i.v. (D) or i.p. (E). After 3 h, phospholipids in each tissue were extracted and analyzed by LC-MS/MS. Data are shown as mean+SD (* p < 0.05, n=5) (F) Schematic model for ENPP6-mediated uptake of GPC in hepatocyte (left) and oligodendrocyte (right).