Abstract
The fate of developing T cells is specified by interactions of their antigen receptor with self-peptide/MHC complexes displayed by thymic antigen presenting cells (APCs). Various thymic APCs subsets are strategically positioned in particular thymic microenvironments and orchestrate the selection of a functional and self-tolerant T cell repertoire. Here, we will review the different strategies that these APCs employ to sample and process self-antigens and thereby generate partly unique, ‘idiosyncratic’ peptide/MHC ligandomes. We will discuss how the particular composition of these APC-subset-specific peptide/MHC ligandomes not only shapes the T cell repertoire in the thymus, but may also indelibly imprint the behavior of mature T cells in the periphery.
The recognition of self-peptides that are embedded in major histocompatibility complex (MHC) molecules on thymic antigen-presenting cells (APCs) is critical for determining the fate of developing αβ T cells. Somewhat paradoxically, recognition of self can elicit diametrically opposed outcomes. On one hand, it is essential for thymocyte survival and commitment to either the CD4+ or CD8+ T cell lineage (that is, for positive selection of thymocytes). On the other hand, recognition of self can be a death verdict for thymocytes, mediating the negative selection of these cells, or it can skew cells to alternative fates, such as regulatory T (TReg) cell differentiation. The classical affinity model of thymocyte selection offers an attractive conceptual framework to resolve this apparent contradiction (Box 1). However, it does not take into account the fact that positive and negative selection largely occur in discrete thymic microenvironments, namely the cortex and the medulla, respectively. Both compartments contain selection niches composed of different types of APCs (Figure 1), thereby providing microenvironments that orchestrate a spatial and temporal segregation of thymocyte selection. In this Review, we will focus on recent advances in our understanding of key features of individual thymic APC subsets and discuss how these relate to the generation of a functional and self-tolerant αβ T cell repertoire.
Box 1 | The affinity model of thymocyte selection (with ‘Figure Box 1‘).
This model centres on the strength of the TCR's interaction with self peptide-MHC complexes as a critical cell fate determinant. Weak interactions are required to protect thymocytes from ‘death-by-neglect’ and to promote the positive selection of naïve T cells. Strong interactions cause negative selection by apoptosis. TCR transgenic studies showed that negative selecting peptides are primarily high affinity agonists (including cognate ligands, i.e. peptides that lead to activation of mature T cells), while positive selecting peptides are often low affinity antagonists or weak agonists (often called altered peptide ligands). Interestingly, in TCR transgenic systems, high affinity agonists were also shown to cause clonal deviation, that is, the re-direction of autoreactive T cells into to the TReg cell lineage. A surprisingly broad range of affinities seem to be permissive for TReg cell differentiation, and as affinity rises, the propensity to generate TReg cells increased. Thus, a modified version of the affinity model, where TReg cell differentiation occurs optimally within a window between positive and negative selection, is currently favored. Of note, the demarcation between clonal deletion and clonal deviation is remarkably plastic and seems to be subject to stochastic influences. For example, in TCR transgenic systems, intrathymic expression of an agonist ligand can concomitantly promote either TReg cell or naïve cell fates in some cases, or both TReg cell differentiation and deletion in other cases. Not surprisingly then, the natural repertoire of TCRs expressed by naïve CD4 T cells and TReg cells shows some overlap. Mathematical modelling suggests that distinct fates can arise from one receptor's interactions with the same ligand by iterative summing of TCR signals during multiple interactions, or by changes (‘developmental tuning’) of thymocyte sensitivity over time 106.
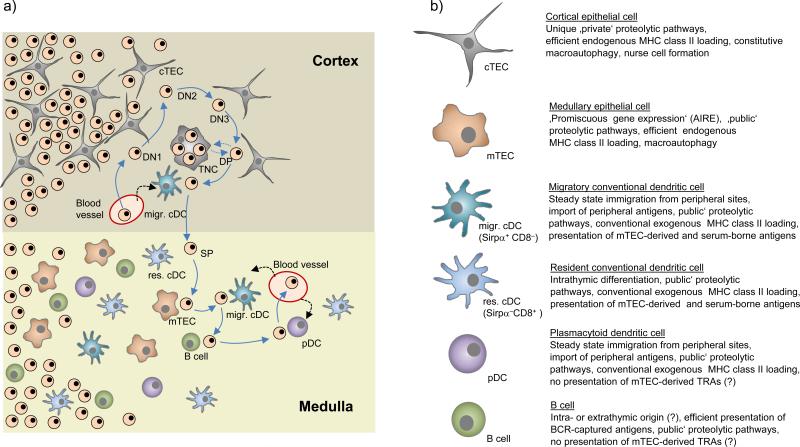
Figure 1. Stromal cell interactions during T cell development.
(a) Successive stages of double-negative (DN) T cell development are accompanied by an outward movement of thymocytes towards the sub-capsular zone. Subsequent to β-selection at the DN3 stage, double-positive (DP) cells ‘randomly walk’ through the outer cortex, which possibly facilitates the ‘scanning’ of cortical thymic epithelial cells (cTECs) for positively selecting ligands. At this stage, DP thymocytes may be engulfed by cTECs and form so-called thymic nurse cells (TNCs), whereby the molecular control and physiological relevance of this process remains to be established. Interactions of DP cells with cortical conventional dendritic cells (cDCs) may lead to negative selection. It remains open whether these cortical cDCs exclusively belong to the migratory Sirpα+ subset. Positively selected, CD4 or CD8 lineage-committed thymocytes relocate into the medulla by directed migration. Upon reaching the medulla, single-positive (SP) cells again assume a ‘random walk’ motion pattern. Through this random migration, SP cells may now ‘scan’ resident (res.) and migratory (migr.) cDCs, medullary thymic epithelial cells (mTECs), plasmacytoid dendritic cells (pDCs) and B cells. It is estimated that SP cells engage in around five contacts with antigen presenting cells (APCs) per hour, so that over their 4-5 days residency in the medulla, T cells may serially interact with several hundred APCs. (b) Key functional properties of thymic APCs discussed in this Review.
Antigen presentation in the cortex
At the peak of its productivity, the mouse thymus each day generates around fifty million CD4+CD8+ double positive (DP) thymocytes that audition for selection1. More than 90% of these precursors are subject to death by neglect, as they express ‘useless’ T cell receptors (TCRs) that do not mediate positive selection. Positive selection of ‘mainstream’ αβ T cells is contingent upon permissive interactions with a single APC type, namely cortical thymic epithelial cells (cTECs). For conceptual clarity, we will therefore restrict a more detailed discussion of antigen presentation in the cortex to cTECs and their role in positive selection, and will only briefly touch upon negative selection in the cortex at the end of this section.
Cortical epithelial cells
cTECs are arranged in a three dimensional scaffold that supports intimate interactions with double negative (DN) and DP thymocytes. In addition, individual cTECs can form multi-cellular complexes that encompass up to 20 thymocytes and are referred to as thymic nurse cells (TNCs). TNC numbers are decreased in TCR-transgenic mice, possibly as a consequence of ‘facilitated’ transit of thymocytes through β-selection and positive selection 2. Thus, it seems that TNC formation is not essential for T cell development per se, but may result from lengthy ‘audition’ events that occur when only a small subset of DP thymocytes meets the positive selection criteria. Consistent with this, in non-TCR transgenic mice, TNCs were enriched in thymocytes harbouring secondary TCRα rearrangements2. Whether such unusual selection niches are indeed required to promote thymocyte survival and/ or continued TCR rearrangements remains to be shown.
Why is positive selection crucially dependent on a single stromal cell type, when tolerance, as discussed further below, can be mediated by a variety of cell types? One might assume that the essential function of cTECs simply depends upon their location and abundant surface expression of MHC molecules. However, this is not the case. Instead, it is becoming increasingly clear that the crucial role of cTECs is, at least in part, a result of the unique machineries that these cells use to process antigens. It is likely that these proteolytic pathways (Figure 2) – discussed in detail in a previous review 3 – endow cTECs with a largely unique peptide–MHC (pMHC) ligandome that is distinct from that displayed by any other thymic or peripheral APC.
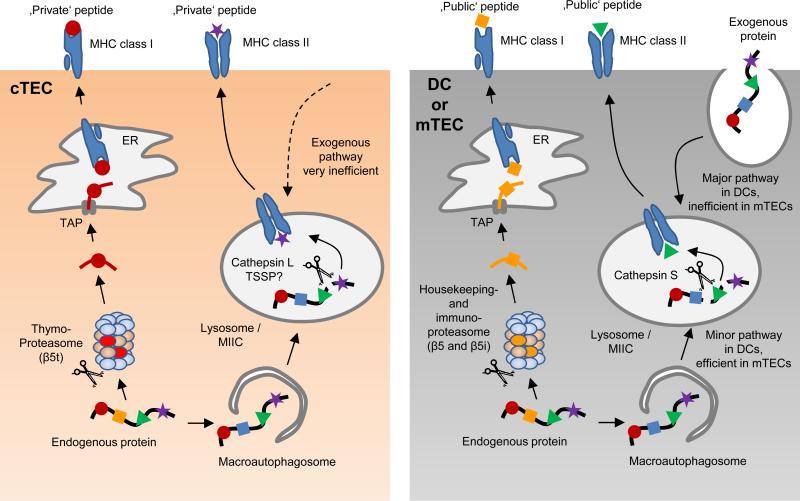
Figure 2. Unique proteolytic pathways generate ‘private’ MHC-bound peptides in cTECs.
Processing of a given endogenous protein substrate by cTECs may give rise to unique, ‘private’ peptides, which differ from ‘public’ peptides generated by mTECs and DCs. MHC class I-bound peptides on the surface of cortical thymic epithelial cells (cTECs) are predominantly processed by proteasomes containing the catalytic subunit β5t (so called thymoproteasomes). Due to a distinct proteolytic activity of the thymoproteasome, this is likely to lead to the generation of cTEC-specific, ‘private’ peptide epitopes that differ from ‘public’ epitopes generated by mTECs or DCs through the housekeeping proteasome or the immuno-proteasome. MHC class II-bound peptides on cTECs seem to be mostly derived from an unconventional, endogenous MHC class II-loading pathway that involves the macroautophagy-mediated shuttling of cytoplasmic proteins into lysosomes. In this proteolytic compartment, processing by the proteases cathepsin L and thymus-specific serin protease (TSSP) may generate unique ‘private’ peptides. MHC class II-bound peptides on mTECs may likewise be mostly derived from macroautophagy–mediated endogenous MHC class II-loading; however, the lysosomal proteases that generate MHC class II-bound peptides in mTECs differ from those in cTECs, being essentially identical to those used by DCs for the processing of exogenously-derived substrates along the ‘conventional’, exogenous MHC class II pathway. Of note, it is likely that the pMHC ligandome of cTECs represents a mixture of ‘private’ and ‘public’ peptides that are uniquely present on cTECs or shared with other APCs, respectively (see Figure 4).
Antigen processing in cTECs
In terms of MHC class I antigen presentation, cTECs express a unique catalytic subunit of the proteasome referred to as β5t. Proteasomes that incorporate β5t are referred to as ‘thymoproteasomes’. They have a substrate preference that is distinct from proteasomes containing the β5 or β5i subunits 4 (termed ‘housekeeping proteasomes’ and ‘immunoproteasomes’, respectively). Mice lacking thymoproteasomes show a substantial defect in positive selection of CD8+ T cells 5.
In terms of MHC class II antigen presentation, cTECs express the unique lysosomal proteases cathepsin L and thymus-specific serine protease (TSSP). Deficiency in these proteases results in impaired selection of CD4+ T cells. Cathepsin L-deficient mice show a strongly decreased polyclonal CD4+ T cell repertoire in the thymus 6, whereas TSSP deficient mice have normal polyclonal CD4+ T cell numbers, yet display defective positive selection of certain MHC class II-restricted transgenic TCRs as well as altered antigen-specific CD4+ T cell responses 7. Moreover, cTECs display an unusually high rate of constitutive macroautophagy, a mechanism that can support the ‘unconventional’ loading of peptides onto MHC class II molecules via an endogenous route 8. Positive selection of several MHC class II-restricted transgenic TCRs was altered upon interference with macroautophagy in thymic epithelium, consistent with the idea that autophagy shapes the MHC class II ligandome of cTECs 9.
Bearing in mind that the avidity/affinity model of thymocyte selection does not envisage any need for unique positively selecting peptides, why may these distinct processing pathways have evolved? Do they generate ‘private’ peptides that are exclusively displayed by cTECs and that have unique properties required for positive selection? Or do these peptides simply dilute ubiquitous ‘public’ peptides, which are nonetheless the major mediators of positive selection? Alternatively, do peptides on cTECs merely have to be different from those presented by other thymic APCs? The latter proposition is supported by the finding that the reconstitution of cathepin L–deficient mice with MHC class II–/– bone marrow, which abrogates negative selection of CD4+ T cells by hematopoietic APCs, largely rescued their CD4+ T cell compartment 10. This indicates that positive selection of CD4+ T cells by Cathepsin L-deficient cTECs is not per se inefficient; however, an unusually large fraction of cells selected in this way are subject to negative selection. Hence, positive selection on different (but not functionally unique) ligands might be necessary to prevent a disproportionate loss of T cells due to subsequent re-encounter of the very same peptides that mediated positive selection in a ‘negatively selecting setting’, that is, on medullary APCs that express abundant co-stimulatory molecules 3. Nevertheless, several observations concerning the role of the thymoproteasome for the selection of CD8+ T cells suggest a different scenario. Thus, neither the reconstitution with MHC class I-deficient bone marrow cells nor the inactivation of Bim rescued the CD8+ T cell compartment of thymoproteasome-deficient mice 11, 12. Therefore, the role of thymoproteasome-dependent peptides cannot be to avert excessive thymocyte deletion. Gene-replacement experiments provide further evidence for the notion that it is the actual nature of the peptides generated by the thymoproteasome, rather than a mere difference between the pMHC repertoires of cTECs and other APCs, that matters. By inserting β5i into the β5t gene locus in β5i–/– mice, animals were engineered in which, independent of β5t, the MHC class I ligandomes differed between cTECs and other APCs (in this case shaped by the immunoproteasome versus the housekeeping proteasome, respectively) 12. This difference alone did not restore positive selection in these animals; by inference, peptides generated by β5t-containing thymoproteasomes are not only different, but may somehow bear unique biophysical features related to positive selection.
The putative significance of ‘private’ peptides
How could ‘private’ peptides on cTECs be specialized for positive selection? They might bind MHC molecules more weakly, as suggested by the observation that β5t-containing proteasomes, in contrast to those harbouring β5 or β5i, inefficiently cleave substrates adjacent to hydrophobic amino acids 5, 13. MHC class I molecules preferentially bind peptides with hydrophobic C-termini. Therefore, wobbly binding of β5t-derived peptides might result in a faster TCR off-rate and thereby promote positive selection, a scenario similar to the generation of partial agonists by altering the MHC anchor residues of immunogenic peptides 14. Although attempts to compare the stability of pMHC complexes on cTECs with that on other APCs have so far failed to disclose such differences 11, 12, there is independent evidence that β5t engenders a bias towards ‘weak’ interactions for positive selection. CD5 expression-levels on SP thymocytes are thought to reflect the signalling intensity of the positively selecting TCR–pMHC interaction, and ‘tuned’ CD5 levels persist on mature peripheral T cells as a footprint of thymic selection 15. Intriguingly, the diminished CD8+ SP compartment found in β5t–/– mice is mostly composed of cells expressing elevated levels of CD5 and also Nr4a1, suggesting that positive selection in the absence of β5t mostly entails interactions of relatively higher affinity 12. In the same vein, TCR transgenic studies showed that selection of ‘natural’ CD5low clones, such as CD8+ T cells expressing the HY TCR, is highly dependent on β5t, whereas selection of CD5hi clones, such as those expressing the OT-I TCR, is not, although amongst five different TCR transgenics the extent of β5t dependency did not show a perfect inverse correlation with CD5 expression levels 11. Thus, thymoproteasome-derived peptides, and possibly private peptides generated through other cTEC-specific pathways in general, might favour selection of CD5lo T cell clones.
Selection of TCRs across an affinity range for self
It has been suggested that the re-exposure of mature T cells to their positively selecting peptide(s) is necessary for homeostasis through continual tonic TCR stimulation16. According to this scenario, T cells selected on ‘private’ pMHC ligands that are not re-encountered outside the thymus are predicted to have a competitive disadvantage during steady state homeostasis. Consistent with this idea, mature CD5low T cells in secondary lymphoid tissues are indeed less responsive to homeostatic cytokines when compared to their CD5hi counterparts17, 18. In further support of such a link between thymic pMHC-experience and mature T cell homeostasis, CD5low T cells expressing the β5t-dependent HY TCR are notoriously poor at homeostatic proliferation, whereas CD5hi cells expressing the OT-I TCR, which is selected fairly efficiently in the absence of β5t, show robust homeostatic expansion 11. Also, TCRs of CD5low cells, in distinction from those of CD5hi cells, are less ’pre-loaded’ with basal phosphorylation of TCRς, which might put them at a competitive disadvantage in responding to foreign antigens16, 19. Indeed, in several infection models in which polyclonal CD4+ T cell responses to pathogens were examined, CD5hi T cells out-competed CD5low T cells. This observation lead to the suggestion that the raison d'etre of positive selection, rather than imprinting self-MHC restriction, is to bias T cell selection towards strongly self-reactive clones endowed with a homeostatic advantage and a head start in anti-pathogen responses 19. Hence, the idea that private peptides serve the purpose of skewing positive selection towards CD5low T cells that weakly respond to self may appear counter-intuitive.
CD5low cells nonetheless do constitute a considerable fraction of the steady state T cell repertoire 19. So why would this be beneficial or even necessary for a functional immune system? First, a repertoire solely composed of clones with high self-reactivity might be prone to incite autoimmunity. However, there is as yet no evidence to support this notion. For instance, β5t–/– mice display intact negative selection 11 and do not exhibit any signs of autoimmunity. Second, the presumed competitive disadvantage of CD5low clones selected through low-affinity interactions may in fact not be a general rule. Persaud et al. compared two CD4+ T cell clones with identical affinity for a Listeria antigen, one presumably selected and maintained by weak interactions with self, as inferred from its CD5lo phenotype, the other by stronger interactions, i.e. being CD5hi and displaying higher TCRς phosphorylation 20. This alleged differential signalling strength during positive selection correlated with differences in basal TCR signalling in mature peripheral cells. Thus, the CD5hi clone was poised to produce more IL-2, whereby this property, consistent with the ‘tonic signalling’ hypothesis, was actively maintained in the periphery through recognition of unknown self-pMHC ligands. Regardless of this difference, in the course of a Listeria infection, CD5low and CD5hi T cells showed comparable proliferative responses, and the CD5hi clone eventually underwent more extensive cell death, so that in this case the CD5low clone ultimately dominated the immune response.
Taken together, unusual antigen processing in cTECs seems to diversify the T cell repertoire for maximal versatility, as best exemplified by the thymoproteasome and CD8+ T cell selection. Interference with this cTEC-specific pathway of pMHC generation results in a ‘crippled’ CD8+ T cell repertoire that seems dominated by T cells with higher affinity for self antigens. Corresponding consequences of Cathepsin L- or TSSP-deficiency for the peripheral CD4+ T cell repertoire have yet to be described.
Negative selection in the cortex
As pointed out in the beginning, the vast majority of thymocyte death in the cortex can be attributed to failure of a large fraction of DP cells to undergo positive selection 21. Nonetheless, there is also a substantial loss of DP thymocytes through negative selection. Recent data show that the number of thymocytes dying through negative selection in the cortex is in fact much higher than previously appreciated and may even exceed the number of cells that pass through positive selection 22, 23. Using a TCR signalling reporter to identify thymocytes that were rescued from deletion in mice lacking Bim, it was estimated that 5 × 105 cells per day undergo negative selection in the cortex 23. This figure not only exceeds the estimated number of positively selected cells, but is also around two-fold higher than the number of cells believed to undergo negative selection in the medulla.
Intriguingly, cortical negative selection of thymocytes specific for ‘ubiquitous’ self-antigens was shown to depend on a crucial contribution of dendritic cells (DCs). The heterogeneity and functional attributes of thymic DCs will be discussed in the section on medullary APCs. At this point, it may suffice to highlight that the critical role of DCs in cortical negative selection is all the more remarkable considering that there are very few DCs in the cortex compared with the medulla and because ‘ubiquitous’ antigens are predicted to also be displayed by cTECs 24. Possibly, these observations reflect an inherent inefficacy of cTECs to support negative selection. Consistent with this, imaging analyses of cortical negative selection in situ revealed that thymocytes arrest and signal adjacent to DCs, even when antigen is also displayed by cTECs 25. Because these experiments involved exogenous delivery of agonist peptide, cTEC-specific pathways of antigen processing are unlikely to be the sole determinant of this impaired capacity of cTECs to induce clonal deletion. Future experimentation is needed to assess the contribution of other candidate parameters such as co-stimulation, cell-adhesion and MHC-turnover.
Antigen presentation in the medulla
The medulla serves a crucial function for T cell tolerance induction, as a disarrayed 3D architecture of the medulla, disturbed development of its stromal components, impaired transit of positively selected thymocytes into or premature egress from the medulla all result in spontaneous manifestations of autoimmunity (reviewed in 3, 26). Central hallmarks of the thymic medulla that specify this critical tolerogenic role are on the one hand the ‘ectopic’ expression of a myriad of otherwise strictly tissue-restricted antigens (TRAs) by medullary thymic epitelial cells (mTECs) and on the other hand the unique ensemble of hematopoietic APCs that seed this microenvironment.
Medullary thymic epithelial cells
The phenomenon of promiscuous gene expression in mTECs has been reviewed in detail elsewhere 27, 28. Some salient features of promiscuous gene expression and novel insights are highlighted in Box 2. Although the entire mTEC population collectively expresses almost all ‘peripheral’ transcripts, each TRA is only expressed by a minor fraction (1–3%) of mTECs at any given time (Figure 3). How this mosaic expression pattern ultimately translates into faithful presentation of thousands of self-antigens in a way that ensures efficient tolerance remains puzzling.
Box 2 | Promiscuous gene expression.
The term promiscuous gene expression refers to the ectopic expression of otherwise tissue-restricted antigens (TRAs) by medullary thymic epithelial cells (mTECs). Remarkably, besides tissue-specificity, promiscuous gene expression violates other fundamental rules of tightly controlled lineage-specific gene expression such as developmental switches and sex-specificity, thus comprehensively representing the immunological self of peripheral tissues.
The Autoimmune Regulator (AIRE) protein remains the only identified molecular regulator dedicated to controlling a sizeable fraction of the promiscuously expressed gene pool 27. It does not seem to act as a classical sequence-specific DNA-binding transcription factor. Instead, AIRE targets and turns on silent gene-loci by binding to non-methylated H3K4, which marks promoters in closed chromatin regions 107, 108. It apparently acts as a docking platform for different multi-protein complexes known to facilitate transcription by generating local double-strand breaks, promoting mRNA processing and relieving stalled RNA polymerase 109, 110. Yet, none of the presently discussed models of AIRE's function 111 satisfactorily accounts for all the intricacies of promiscuous gene expression, most notably the observation that a given TRA is only expressed by a minor subset of 1-3 % of mTECs at any point in time (see Figure 3). Understanding how this mosaic pattern of promiscuous gene expression is generated and whether it is maintained in time and space at the single cell level will be key to eventually comprehend how central tolerance can be so efficient, sensitive and stringent.
Although it was initially thought that promiscuous gene expression in single mTECs is stochastic 52, 54, a recent study revealed distinct and fluctuating patterns of gene co-expression in subsets of human mTECs, implying that mTECs transiently display TRAs in certain linkage groups 53. Co-expression groups ranged between approximately 100 and 300 TRAs, preferentially mapping to particular chromosomes whereby co-expressed gene loci co-localized within nuclear subdomains, pointing to a level of regulation at the higher genome order.
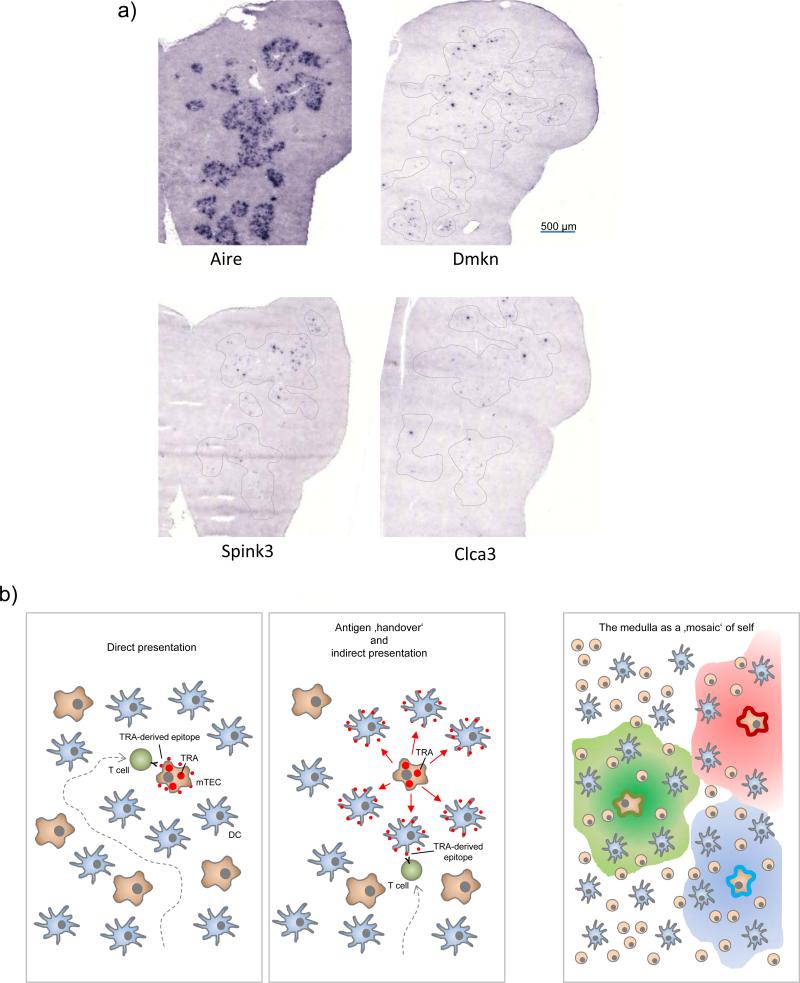
Figure 3. Topological aspects of ‘promiscuous gene expression’ and direct versus indirect presentation of TRAs.
(a) Ttissue-restricted antigens (TRAs) are expressed by only a small subset of mTECs at any given point in time: In-situ hybridization of a section through an entire thymic lobe for Aire mRNA expression (left) shows that Aire-expressing cells are densely packed in medullary regions. By contrast, transcripts of three representative TRAs (dermakine, Dmkn; Serin protease inhibitor kazal type 3, Spink3; chloride channel calcium activated 3; Clca3) are only detectable in very few cells that are scattered throughout medullary areas (dotted lines). (b) Tolerogenic presentation of TRAs that are expressed by few mTECs may occur in two not mutually exclusive ways: (left) direct presentation by TRA-expressing mTECs themselves, whereby efficient endogenous MHC class II-loading by mTECs in conjunction with serial ‘scanning’ of multiple medullary APCs by thymocytes increases the likelihood of cognate self-antigen interactions. (middle) ‘Antigen handover’ to neighbouring cDCs may extend the area of tolerogenic presentation in a mosaic fashion beyond the topologically restricted expression pattern (right). The mechanistic details of this ‘directional antigen transfer’ transfer remain to be established. It is conceivable that TRAs are released or shed in soluble form to be subsequently captured and processed by cDCs for presentation on MHC class I or II. Apoptosis of terminally differentiated mTECs may lead to the release of apoptotic fragments that can also transfer mTEC-derived self-antigens to cDCs. In addition, functional peptide-MHC ligands are unidirectionally translocated from mTECs to DCs 42, 118.
Self antigens expressed by mTECs may be seen by T cells in two ways (Figure 3): first, through ‘autonomous’ presentation by mTECs themselves or, second, through antigen hand-over and presentation by neighbouring APCs. Direct presentation of endogenously expressed antigens by mTECs can not only induce negative selection of CD8+ T cells 29, 30 but also efficiently elicits CD4+ T cell tolerance 31-34. At the same time, mTECs are conspicuously inefficient in ‘conventional’ MHC class II presentation of extracellular substrates 35, 36. Hence, mTECs apparently evolved strategies to bypass the classical exogenous pathways of MHC class II loading in order to focus their MHC class II-ligandome on endogenous self-antigens.
Endogenous MHC class II loading in mTECs
How do mTECs load MHC class II molecules with intracellular antigens? Candidate pathways fall into two categories (reviewed in 8). The first comprises proteasome- and TAP-dependent mechanisms, implying a leakage of the ER-content into MHC class II loading compartments. The second category comprises processes collectively known as autophagy (’self eating’): microautophagy, chaperone-mediated autophagy and macroautophagy. Their common principle is the delivery of cytoplasmic constituents to lysosomes, which presumably intersect with the MHC class II loading pathway 37. So far, only the role of macroautophagy has been examined in the context of thymocyte selection. Athymic nude mice grafted with macroautophagy-deficient thymi displayed various manifestations of immune-mediated tissue-damage, consistent with a crucial function of macroautophagy in TECs for loading peptides onto MHC class II molecules for T cell repertoire selection 9. However, these studies left open whether the observed symptoms actually reflected a failure of negative selection by mTECs or were driven by impaired positive selection by autophagy-deficient cTECs, two not mutually exclusive possibilities.
More recent work provided compelling evidence that macroautophagy indeed supports tolerogenic endogenous MHC class II loading in mTECs. When two closely related model antigens were targeted to the cytosol of mTECs, a variant that was earmarked for autophagosomal degradation was presented with much higher efficacy and displayed a superior capacity to induce negative selection of CD4+ T cells 38. The same study also showed that a mitochondrial version of a model-antigen did require macroautophagy for tolerogenic presentation by TECs, whereas direct presentation of a membrane-bound form of the same antigen was macroautophagy-independent 38. Possibly, endogenous access to MHC class II of substrates residing in the cytoplasm or within organelles, such as mitochondria, peroxisomes or the nucleus, may generally require macroautophagy, consistent with the role of autophagy in sampling these sub-cellular compartments 39. By contrast, membrane proteins seem to be inherently prone to access MHC class II loading compartments independently of macroautophagy 40.
Direct versus indirect presentation of self antigens by mTECs
A clear delineation of the quantitative or qualitative impact of direct versus indirect presentation of TRAs by mTECs or DCs (or any other thymic APC for that matter), respectively, is only slowly emerging, partly due to potential redundancies between the two mechanisms. Relying on transgenic neo-self antigens, there is a wealth of information supporting the idea that direct presentation by mTECs is an exquisitely efficient tolerance mechanism (reviewed in 41). At the same time, there is accruing evidence that the medulla provides a specialized micro-milieu conducive to intercellular antigen transfer 42. Yet, few experimental models document an essential requirement for such antigen hand-over, and some of these findings remain controversial 29, 43. In a recent study, MHC class II-tetramers were employed to monitor steady state negative selection of polyclonal CD4+ T cells reactive to interphotoreceptor retinoid-binding protein (IRBP), an AIRE-dependent TRA exclusively expressed by mTECs. Ablating MHC class II expression in hematopoietic cells abolished negative selection of T cells specific for this physiologically expressed self antigen, indicating an essential requirement for intercellular transfer between antigen-expressing mTECs and antigen-presenting hematopoietic APCs, at least for certain epitopes of IRBP 44.
A conclusive dissection of the dual role of mTECs (as antigen providers and presenters) in tolerizing the polyclonal T cell repertoire remains experimentally challenging. Selective ablation of either MHC class I or MHC class II expression on mTECs by conditional gene targeting has been surprisingly difficult to achieve. A further caveat of such an approach is that MHC class II-dependent ‘thymic crosstalk’ between thymocytes and mTECs organizes mTEC differentiation 45, so that abolition of MHC class II on mTECs will most likely affect promiscuous gene expression in qualitative or quantitative terms. In order to avoid such confounding effects an experimental strategy of tissue-specific knockdown of MHC class II molecules in transgenic mice (termed C2TAkd mice) has been devised 31. The selective attenuation of antigen presentation by mTECs in these mice resulted in sporadic bouts of mild tissue infiltrations, yet did not elicit overt autoimmunity. These findings contrast with the spontaneous autoimmunity ensuing from AIRE-deficiency or even from selectively abrogating the expression of single TRAs in mTECs 46, 47. At first glance, this could be interpreted to indicate that direct antigen presentation to CD4+ T cells by mTECs, in contrast to TRA expression, is not essential to prevent autoimmunity; however, it is equally possible that the residual MHC class II expression on mTECs in C2TAkd mice might still suffice to censor auto-reactive CD4+ T cells at the high affinity-end of the TCR spectrum.
In further support of a substantial autonomous contribution of mTECs as APCs for negative selection of polyclonal CD4+ T cells, the CD4+ SP thymocyte compartment in C2TAkd mice was markedly enlarged. In fact, when compared, the decreased expression of MHC class II molecules on mTECs in these mice and the complete ablation MHC class II expression on DCs in MHC class II–/– → WT BM chimeras had a similar impact on the degree of negative selection within the CD4+ SP thymocyte compartment 31. Moreover, combining hematopoietic MHC class II deficiency with MHC class II reduction on mTECs had an additive effect, suggesting a non-redundant contribution of both DCs and mTECs to negative selection.
Given the low frequency of mTECs that express and hence potentially present a given TRA 1, ‘saturating’ tolerance induction through direct presentation would require exceedingly efficient scanning of numerous mTECs by thymocytes. Real-time imaging of thymocyte motility showed that this is indeed the case: SP thymocytes ‘randomly walk’ within medullary areas at a velocity of 10 μm/min, allowing them to engage in multiple contacts with APCs48-50. Estimates of the number of APCs that can be scanned within the 4-5 day sojourn of SP cells in the medulla vary from a few hundred to several thousand 1, 49, 51. Bio-informatic modelling based on available TRA (co-)expression data at the single-cell level 52-54 predicts that 200 to 500 mTECs will be sufficient to cover the full TRA repertoire at a given point in time (B.K., H. Mayer and S. Pinto). Shifting TRA expression patterns over time and corresponding fluctuations in the pMHC ligandome of individual mTECs would further reduce the minimal number of cells that need to be scanned, provided that T cells re-encounter the same mTEC over time 49, 53. Notwithstanding a considerable error margin in these calculations, it seems that T cells may not even need to roam through large volumes of the medulla in order to saturate TRA encounters resulting from autonomous presentation by mTECs.
Thymic dendritic cells
The overall contribution of DCs to the total thymic cellularity is in the order of 0.5%. Thymic DCs can be subdivided into three major subsets 55, two of which belong to the conventional (also known as classical) DC (cDC) lineage, whereas the remaining third of thymic DCs belongs to the plasmacytoid DC (pDC) lineage. The heterogeneity of DCs in the thymus raises obvious issues as to a possible functional specialization of individual subtypes. Determinants of such a division-of-labour could be cell-biological features pertaining to APC function (antigen uptake and processing), intra- versus extra-thymic origin and the positioning within distinct thymic microenvironments. All these features will eventually define the sampling territories of each subset and hence its self peptide–MHC ligandome.
Resident versus migratory cDCs
Around two thirds of thymic DCs can be classified as CD11chiCD45RA– cDCs. These can be further subdivided according to differential co-expression of CD8α and SIRPα, with roughly two thirds of thymic cDCs displaying a CD8α+SIRPα– and one third a reciprocal CD8α–SIRPα+ surface phenotype 55. The major CD8α+SIRPα– cDC subset originates from an intrathymic differentiation pathway, and hence these cells are commonly referred to as ‘resident’ cDCs, whereas the minor CD8α–Sirpα+ cDC subset is maintained by steady state immigration from the periphery, and these cells are therefore referred to as migratory cDCs 56.
Resident cDCs in the thymus bear obvious phenotypic resemblance to CD8α+ cDCs in the periphery. The latter are known to be particularly efficient in cross-presentation, that is, the presentation of exogenous antigens in the context of MHC class I 57. Thymic CD8α+SIRPα– cDCs indeed also showed a superior cross-presentation capacity in vitro when compared to the migratory subset 58. In vivo, intrathymic cross-presentation was found to contribute to CD8 T cell tolerance towards a model-antigen mimicking a TRA-like expression pattern in mTECs 29; since these studies did not address the identity of the cross-presenting cell type, it remains to be established whether there is a differential contribution of resident versus migratory cDCs in this context.
Although, on the whole, DCs are markedly more abundant in the medulla than in the cortex, it is unclear whether this applies in equal terms to both migratory and resident cDCs. Recent work has identified the chemokine XCL1 (also known as lymphotactin) as a crucial determinant of the medullary localization of cDCs 59, as Xcl1-deficient mice have fewer medullary cDCs. Although not directly addressed in this study, the fact that only CD8α+ cDCs express the receptor for XCL1 (XCR1) suggests that this mis-localization primarily affects resident, but not migratory, cDCs. As mTECs are the only thymic stromal cells producing XCL1 (notably in an AIRE-dependent manner), the XCL1–XCR1 chemokine axis may orchestrate the localisation of resident cDCs next to mTECs. Such a close apposition should facilitate the transfer of mTEC-derived TRAs to DCs, although this scenario still awaits experimental proof.
The migratory CD8α–Sirpα+ cDC subset seems to be guided by different cues. Thus, CCR2-deficient mice showed a selective diminution of migratory DCs in the thymus60, whereby CCR2 signalling seems crucial for the mobilization of peripheral SIRPα+ cDCs rather than their final intrathymic positioning. The same report showed that migratory cDCs can accumulate in the cortex in the vicinity of small vessels and inside perivascular regions, whereas other investigators found that SIRPα+ cDCs preferentially localized near blood vessels at the cortico-medullary junction and within deeper regions of the medulla (D. Atibalentja and E. Unanue, personal communication). Notwithstanding these apparent discrepancies, there is some consensus that SIRPα+ migratory cDCs more efficiently sample intravenously injected model antigens from the bloodstream in vivo when compared with resident cDCs 35, 60-62.
Taken together, the differences between resident and migratory cDCs in their origin, responsiveness to chemokines and selective occupancy of microenvironmental niches imply a degree of functional specialization. Resident cDCs preferentially seed the medulla and seem best equipped to focus their antigen display on self antigens captured within the thymic microenvironment. By contrast, migratory cDCs are found both in the medulla and the cortex and may serve a dual role: first, to transport peripherally acquired self antigens into the thymus and, second, to capture and present blood-borne self antigens. It is unlikely, however, that there is a strict demarcation between these functions.
Plasmacytoid dendritic cells
Roughly one third of all thymic DCs are plasmacytoid Dendritic Cells (pDCs). pDCs enter the thymus as a migratory population from peripheral sites 56, indicating a close lineage relationship between peripheral and thymic pDCs. As peripheral pDCs serve a crucial function in the protection against viral infections through their production of type I interferons63, the presence of a pDC population in the thymus was suggested to reflect a similar innate immune function in a primary lymphoid organ 55.
Given the poor antigen presentation capacity of pDCs 64, a role in central tolerance had been considered rather unlikely; however, recent data suggest that this view may need to be revised. First, in a reductionist in vitro setting, thymic pDCs were capable of presenting peptide antigen to specific thymocytes and promoting their differentiation into TReg cells65. Second, pDCs were reported to be surprisingly efficient in picking up soluble or particulate model-antigens at peripheral sites in vivo and transporting them into the thymus56, 66. Interestingly, the chemokine receptor CCR9, which controls T progenitor homing to the thymus, is also crucial for pDC recruitment to the thymus 66. As a subset of CCR9+ immature pDCs in peripheral lymphoid tissues was able to promote conversion of naive T cells into TReg cells67, this ‘tolerogenic’ pDC subset may also exert tolerogenic functions upon recruitment into the thymus. Indeed, adoptively transferred OVA-loaded wild-type pDCs, but not Ccr9–/– pDCs, migrated to the thymus, where they specifically located to the medulla and promoted clonal deletion of OVA-specific OT-II thymocytes 66.
Altogether, these new data present a strong case for a contribution of pDCs to central tolerance. Of note, pDCs, in contrast to both subsets of cDCs, do not pick up mTEC-derived antigens (J. Nedjic, T. Yamano, J. Derbinski, L.K. and B.K., unpublished observations), indicating that they may sample self antigens in the periphery and then ‘freeze’ their antigen cargo. Moreover, activation of TLRs prevents both cDCs and pDCs from migrating to the thymus68,66, thereby conceivably preventing central tolerance to pathogens under inflammatory conditions. Finally, considering that CCR9 also promotes migration to the intestine, CCR9+ pDCs may not only sample bona fide self antigens but also innocuous foreign antigens, such as food components or constituents of the commensal microflora. However, there is as yet no experimental data to support this intriguing scenario.
Thymic B cells
Approximately 0.3% of thymic cells are B cells, a figure similar to that seen for DCs. The origin of thymic B cells is still a matter of debate; it is unclear whether they derive from intrathymic B lymphopoiesis and/or immigration of peripheral B cells 69, 70. The phenotypic and functional attributes of thymic B cells closely resemble those of conventional B cells (that is, B-2 cells) found in the periphery 71, 72. However, compared with splenic B cells, thymic B cells show elevated expression of MHC class II and co-stimulatory molecules, indicative of potent antigen presentation capacity. Indeed, thymic B cells have repeatedly been found to be capable of inducing negative selection. Most convincingly, myelin oligodendrocyte glycoprotein (MOG)-specific CD4+ thymocytes were negatively selected when an epitope of MOG was exclusively presented by B cells 73.
However, it is presently unclear how thymic B cells fare in their overall contribution to tolerance when compared with DCs and mTECs. For instance, deletion of superantigen-reactive CD4+ T cells was more efficiently induced by DCs, and in the same context B cells entirely failed to negatively select CD8+ thymocyte 74. Consistent with these in vivo data, selective supplementation of reaggregate thymus organ cultures (RTOCs) with different thymic APCs in the presence of soluble OVA peptide resulted in negative selection of OVA-specific CD4+ thymocytes when resident or migratory cDCs were used as APCs, but not when B cells were the APC 75.
Relatively little is known about the parameters that may shape the pMHC-ligandome of thymic B cells. Traditionally, peripheral B cells are considered poor presenters of exogenous antigens. This may also be the case for thymic B cells, explaining their poor performance in the aforementioned studies on negative selection in RTOCs. However, in contrast to the poor efficacy with which B cells present soluble antigens, B cell receptor (BCR)-mediated cognate interactions lead to exceptionally efficient antigen presentation 76. Given the copious amounts of MHC class II expressed by thymic B cells, it is conceivable that B cells may not only present BCR-captured external antigens, but in manner similar to mTECs, may focus their pMHC class II ligandome towards endogenously expressed proteins. This intracellular antigen pool is likely to include germline-encoded or even clonotype-specific regions of the BCR 77. Lack of tolerance towards variable (V)-regions of the BCR caused T cells to provide ‘chronic’ inappropriate help to B cells expressing the respective BCR in a double-transgenic model. This ultimately resulted in systemic autoimmunity78, indicating that robust tolerance towards this special class of self antigens is indispensable.
Cognate T-B interactions are central for germinal centre formation during immune responses to foreign antigens, but may have an intriguing counterpart in the central tolerance process. Thus, BCR-transgenic B cells efficiently mediated negative selection of CD4+ thymocytes expressing a transgenic TCR specific for the same cognate self antigen 72. Although it may be difficult to envision how rare cognate self-antigen-specific B cells within a polyclonal repertoire may be sufficient to impose tolerance, the same study reported that even a polyclonal B cell population mediated a degree of deletion of TCR transgenic CD4+ T cells.
In sum, these intriguing new data should reignite interest in the role of B cells in central T cell tolerance. Thymic B cells may afford another layer of tolerance towards BCR-derived self-constituents, thus pre-emptying the previously described peripheral checkpoints of T cell tolerance to this unique class of self antigens 79. Other relevant issues in this regard are whether the scope of B cell-mediated central T cell tolerance is indeed dictated by the BCR repertoire of thymic B cells, how diverse the thymic BCR repertoire is, and whether auto-reactive B cells may be enriched in the thymus to allow for efficient presentation of soluble self-antigens.
Perspectives
Following our review of key cell biological attributes of the different thymic APC populations in the context of T cell repertoire selection, we will close with some speculative thoughts on how the intrathymic encounter of self (or the lack thereof) may imprint peripheral T cell behaviour, orchestrate dominant versus recessive mechanisms of tolerance and specify targets of autoimmunity.
Positive selection, homeostatic fitness and immunity to pathogens
Our discussion of antigen presentation for positive selection converged on the view that cTECs generate and display functionally and possibly structurally distinct private self peptides that may sustain the selection of T cell clones displaying weak tonic self-reactivity in the periphery. This notion is at odds with the proposition that the very same self peptides that mediate positive selection are also essential for naïve T cell homeostasis in the periphery and act as co-agonists when T cells respond to foreign antigens 16, 80, 81.
How can this apparent discrepancy be reconciled? First, it is possible that the peripheral self peptides supporting homeostasis and co-activation are not identical, but instead functionally equivalent to those supporting positive selection. Second, one may argue that the functional competence of the peripheral T cell repertoire requires a balanced distribution of clones covering a relatively wide range of tonic self-reactivity, as represented by CD5low and CD5hi T cells. Possibly, a corresponding mix of private and public MHC ligands on cTECs is a prerequisite to select such a composite of T cell clones with low or high tonic affinity, respectively.
One can envisage a potential benefit of having T cells with a wide range of affinities for self antigens (Figure 4). Following infection with pathogens, T cells with high affinity for self could provide a rapid, yet relatively short-lived initial immune response that is then followed by a sustained response by T cells with lower self affinity. The latter are presumably not only less prone to burn out, but also less likely to cause bystander damage to self tissues. This scenario would fit with the observation that β5t–/– mice, which have a numerically smaller but presumably more strongly self-reactive CD8+ T cell repertoire, die in response to infection with influenza virus11. Yet, since the flu-specific response was not tracked in that study, it remains to be determined whether these CD5hi-skewed CD8+ T cells indeed either collapsed faster, made an over-shooting pathogenic response, or failed to respond to antigen at all. Against this background, it will also be interesting to see whether the duration of infections (chronic versus acute) or the spread of pathogens (systemic versus local) are crucial determinants of the relative contribution of CD5low and CD5hi T cell clones to the immune response to foreign antigens, and how these parameters affect their partitioning into the memory pool.
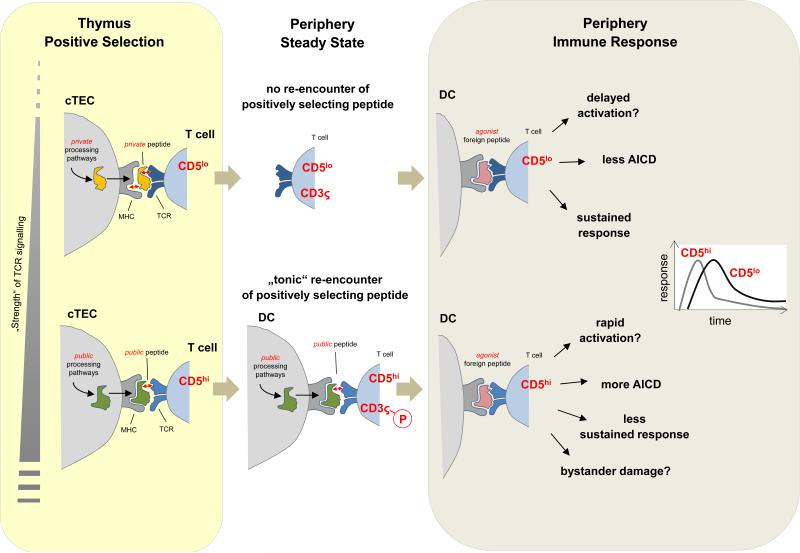
Figure 4. Consequences of positive selection by ‘private’ versus ‘public‘ peptides: a hypothesis.
(Upper panel) ‘Private’ peptides generated through unique proteolytic pathways in cortical thymic epithelial cells (cTECs) may preferentially support selection of CD5low T cell clones via interactions at the lower end of the affinity range that is permissive for positive selection. One determinant of these ‘low strength’ interactions could be that private peptides are weak MHC binders, indicated here by the loose fit between peptide and MHC (red arrow). In the periphery, T cells selected in this way do not re-encounter the positively selecting peptides and hence do not receive tonic signals. As a consequence, their CD3ς chains are not pre-loaded with basal phosphorylation. Yet, it remains possible that CD5low clones receive a degree of tonic input through exposure to cross-reactive ‘public’ peptides in the periphery. (Lower panel) Public peptides may preferentially support selection of CD5hi clones via positively selecting interactions at the relatively higher end of the affinity range. Public peptides might be good MHC binders that generate ‘low strength’ interactions by loosely binding to the TCR (red arrow). In the periphery, continual interactions with the very same peptides support T cell homeostasis and mediate partial CD3ς chain phosphorylation. During an immune response to foreign antigens, CD5low and CD5hi T cell clones of identical specificity may differentially respond with respect to timing and magnitude of clonal expansion and contraction. The dominance of either type of responder might vary with parameters such as duration and anatomical distribution of the infection.
Notwithstanding these considerations, we still lack experimental data to directly link the selection of a given ‘low self-affinity’ TCR-specificity to a specific private peptide the processing of which would be dependent on any of the cTEC-specific pathways of antigen processing. Solving this issue has been hampered by our current ignorance of the identity of the peptides bound to MHC on cTECs. The scarcity of cTECs (1 – 3 × 104 per thymus) renders this a daunting task (Box 3). In this context, the fundamental issue of whether selection of a given TCR specificity actually requires a single, specific self peptide has not been resolved. Likewise, we do not know whether private peptides on cTECs are equally important for CD4+ and CD8+ T cell repertoire selection. This question is all the more interesting since high tonic self-responders among naïve CD4+ T cells seem inherently more prone to undergo peripheral conversion into induced FOXP3+ TReg cells 82.
Box 3 | Towards directly addressing the peptide-MHC ligandome of thymic stromal cells.
Remarkably, one of the first attempts in the early 1990's to assess the peptide (p)MHC ligandome by peptide-elution and -sequencing reported a comparison between cTECs and splenic APCs 112. At the time, only 17 of the most abundant self peptides were identified, from an estimated 2,000 to 10,000 distinct peptides presented by MHC class II or I, respectively. With technology improving, the field has seen increasingly detailed assessments of cell-type specific pMHC ligandomes, particularly of tumor cells. However, rare ex vivo isolated populations such as thymic stromal cells remain a major technical challenge.
In general, naturally processed peptides have been characterized from acid extractions of affinity-purified MHC molecules, which are then sequenced typically by mass spectrometry. This approach was used to describe peptides bound to MHC class I and II molecules in the human thymus 113, 114. However, since whole tissue was used, these studies fell short of assigning the identified peptides to particular stromal cell types. Another recent study identified 50-100 peptides from HLA class II or I molecules from human thymic DCs and compared these to peptides from thymic APCs depleted of DCs 115, giving initial insight into differences in the ligandomes of thymic APCs. The amount of starting material required with this approach (at least 108 cells) so far precludes an informative analysis of MHC bound peptides from rare populations like cTECs. Another complication arises from the possibility that the elusive private self-peptides presumed to be generated by β5t may more weakly bind to MHC Class I molecules and hence might be lost during the immunoprecipitation step. An alternative approach has been reported that characterized peptides directly extracted with mild acid elution from the surface of intact cells. Since this procedure generates a large number of peptides, not just those bound by MHC, the authors employed a bioinformatics comparison to peptides extracted from MHC-deficient cells to assign them as ‘MHC-bound’116. Chemical and metabolic labelling have also been employed to provide quantitative comparisons between two populations, although this has not been applied to the thymus 117.
Modes of central tolerance: deletion versus TReg cell differentiation
A detailed discussion of how antigen presentation in the thymus may intercalate with factors such as TCR affinity, access to cytokines or developmental tuning of thymocyte responsiveness to specify clonal deletion versus clonal deviation into the FOXP3+ TReg cell lineage exceeds the scope of this article (for recent reviews see 83, 84). Here, it may suffice to highlight two pertinent issues.
First, there is no evidence to indicate that any thymic APC subset is specialized to exclusively promote clonal deletion or TReg cell differentiation. Although efficient TReg cell differentiation apparently requires a properly formed medullary microenvironment 85, the overall size of the polyclonal TReg cell pool is barely affected by the specific lack of DCs or subsets of mTECs, or by aberrant MHC class II expression on these cells (reviewed in 86). Thus, thymic APC subsets obviously can compensate for each other as far as filling the physiological TReg niche is concerned. However, considering the evidence for partly non-overlapping pMHC ligandomes on different thymic APCs, we deem it unlikely that this reflects a true redundancy with respect to the level of TCR specificities that are recruited into the TReg cell repertoire. Large scale TCR sequencing of TReg cell repertoires in the above mentioned settings is expected to resolve this issue.
Second, it seems unlikely that any particular modalities of antigen expression, for instance a ‘promiscuous gene expression-like’ mosaic pattern versus ubiquitous expression, will invariably result in either clonal deletion or clonal deviation. The view that the essential role of Aire in mTECs can be solely ascribed to recessive tolerance through negative selection 87 is difficult to reconcile with the ability of mTECs to autonomously induce antigen-specific TReg cells in neo-self antigen transgenic settings 34. A recent study extended the latter finding to a naturally expressed protein by showing that an Aire-dependent self-antigen induced thymic differentiation of cognate TReg cells 88. Intriguingly, TReg cell progenitors of unknown specificity were found to compete for recognition of rare stimulatory TCR self ligands in the thymus 89-91, which is also suggestive of a link between promiscuous gene expression in rare mTECs and TReg cell differentiation. The possibility that expression and presentation of TRAs by very few mTECs may concomitantly induce negative selection and TReg cell induction has obvious implications regarding the issue that interactions of thymocytes with rare APCs presenting a given cognate self antigen perhaps need not be saturating after all, because the occasional escape of autoreactive T cells would be counterbalanced by dominant regulatory mechanisms.
Autoimmunity: what thymocytes may not see
With increasing knowledge of the multi-layered cellular and molecular mechanisms of central tolerance one is bound to ask which parameters are most limiting and pose the highest risk for autoimmunity. Deep sequencing data of the mRNA signature of mTECs indicate that the scope of promiscuous gene expression is even broader than previously revealed by microarray analyses 92. Given this apparently comprehensive intrathymic representation of otherwise tissue-specific transcriptomes, it becomes even more intriguing and informative to know which fraction of self is actually not covered by central tolerance. Any advance in understanding the molecular regulation of promiscous gene expression by AIRE and beyond would help us to understand how the tolerizing MHC-ligandome in the thymus is generated in the first place and, importantly, how dys-regulation at the genetic, epigenetic and biochemical level could undermine central tolerance and thus possibly explain immune pathologies. In fact, there is an increasing number of examples of how ‘holes’ in central tolerance might specify dominant T cell targets in organ-specific autoimmune diseases.
First, very low expression levels of particular self antigens in human mTECs correlate with a high frequency of cognate, auto-reactive T cells and/or autoantibodies in the case of the myosin heavy chain 6 93, 94, an autoantigen in autoimmune myocarditis, or GAD65 95 and ZnT8, both targets in type 1 diabetes (B.K., S. Pinto, R. Mallone, unpublished observations). Genetic polymorphisms in regulatory regions of some autoantigens, which exert subtle effects on the expression level of self antigens in mTECs but not peripheral tissues correlate with susceptibility to organ-specific auto-immunity as shown for insulin and type 1 diabetes 96-98, the alpha-chain of the acetylcholine receptor and myasthenia gravis 99 and the thyroid stimulating hormone receptor and Graves’ disease 100. Second, similar effects are observed when dominant T cell epitopes are only present in peripheral tissues but not in the thymus either due to differential splicing 101, 102 or due to mis-initiation of mRNA transcription in mTECs (S. Pinto, B.K., unpublished observations). Mis-initiated transcription is possibly a more general feature and thus a risk factor of promiscuous gene expression54. Finally, post-translational modifications of auto-antigens affecting T cell epitopes by cell-type specific expression of modifying enzymes in peripheral target tissues but not mTECs may work to the same effect. Examples in this regard are glycosylation of collagen type II and citrullination of aggrecan and vimentin in the case of rheumatoid arthritis 103 or deamidation of insulin in the case of type I diabetes 104.
Conclusions
The affinity model remains a useful framework to conceptualize thymic selection events, and there has been considerable progress in understanding how the TCR relays quantitative differences in signal strength into qualitatively different cell fates (reviewed in 105). Yet, as reviewed here, a coherent model of thymocyte selection will in addition need to consider temporal, spatial and qualitative aspects of self recognition in distinct thymic microenvironments. In terms of positive selection, it remains tantalizing why ‘monogamous’ interactions of developing T cells with a single ‘dedicated’ stromal cell type are so critical, and we have discussed how this crucial role of cTECs may be specified by their ability to generate ‘private’ pMHC ligands. With regard to central tolerance induction, it is intuitively obvious why ‘promiscuity’, not only at the level of TRA expression in mTECs, but similarly in terms of the diverse characteristics and sheer number of contributing APC subsets, is advantageous. Unravelling functional non-redundancies between tolerogenic APCs, at the level of the self antigen spectra that are presented and regarding tolerance mechanisms (deletion versus TReg cell induction), remains a major challenge for future investigations.
Acknowledgements
L.K. receives support from the Deutsche Forschungsgemeinschaft (Collaborative research centre SFB 1054 and grants KL 1228/4-1 and KL 1228/5-1). B.K. was supported by the German Cancer Research Center (DKFZ), the Deutsche Forschungsgemeinschaft (Collaborative research centre SFB 938) and the European Research Council (ERC-2012-AdG). P.M.A. was supported by NIH grant AI-24157. K.A.H is supported by NIH grants AI088209, AI35296 and AI39560.
Glossary terms
- Positive selection
The process by which immature double-positive thymocytes expressing T cell receptors with intermediate affinity and/or avidity for self-peptide–MHC complexes are induced to differentiate into mature single-positive thymocytes.
- Negative selection
(Also known as clonal deletion). The intrathymic elimination of double-positive or single-positive thymocytes that express T cell receptors with high affinity for self antigens.
- Peptide–MHC ligandome
The repertoire of peptides that are bound by MHC molecules.
- Death by neglect
Double-positive thymocytes have a finite life-span of 3 – 4 days. Failure to engage in positively selecting interactions with self pMHC complexes on cTECs within this period result in programmed cell death.
- Bim
BCL-2-interacting mediator of cell death is a pro-apoptotic molecule that is crucial for negative selection.
- β-selection
The pre-TCR-driven process by which double-negative thymocytes that carry a productively rearranged TCR β-chain undergo proliferative expansion and developmental progression.
- Proteasome
The standard proteasome is composed of 14 α and 14 β subunits, of which three, β1, β2 and β5, are involved in peptide-bond cleavage. Interferon-γ induces the expression of the immunosubunits β1i, β2i and β5i that can replace the catalytic subunits of the standard proteasome to generate the immunoproteasome, which has distinct cleavage-site preferences.
- Macroautophagy
The generally nonspecific sequestration of cytoplasm into a double- or multiple-membrane-delimited compartment (autophagosome) of non-lysosomal origin. Certain proteins, organelles and pathogens may be selectively degraded by macroautophagy.
- MHC anchor residues
Amino-acid residues of an antigenic peptide that bind in pockets in the peptide-binding groove of a major histocompatibility molecule and account for much of the binding energy and specificity of binding.
- CD5
A membrane protein that associates with the TCR complex. It modulates the TCR signal transduction cascade through interactions with various kinases and phosphatases.
- Nr4a1
Also known as Nur77. Nr4a1 encodes an orphan nuclear receptor whose expression is up-regulated by TCR signalling in thymocytes and mature T cells.
- HY TCR
An MHC class I-restricted transgenic TCR recognizing a self-antigen encoded on the Y-chromosome.
- OT-I TCR
An MHC class I-restricted transgenic TCR recognizing an Ovalbumin epitope.
- Tonic TCR stimulation
Continuous ‘sub-threshold’ recognition of self-peptide/MHC complexes by mature T cells, resulting in a basal activation state that enables T cells to rapidly respond to foreign antigen.
- Tissue-restricted antigens (TRAs)
Self-constituents encoded by genes which are expressed by only one or few tissue-specific cell lineages as opposed to housekeeping genes. The term TRA is an operational definition based on available expression catalogues, according to which TRAs are expressed in less than 5 tissues of 60 tested.
- Thymic cross-talk
The mutual developmental dependence of the T cell and the stromal cell (i.e. non-T cell) compartments of the thymus, specified by complex receptor-ligand interactions.
- C2TAkd mice
A mouse strain that expresses a designer microRNA targeting the class 2 trans-activator (C2TA) specifically in mTECs. This leads to a reduction of MHC class II expression to about 10% of its physiological levels, while preserving intact mTEC differentiation and TRA expression.
- CCR9
C-C chemokine receptor type 9 is a G protein coupled receptor recognizing the chemokine CCL25 (TECK; thymus expressed chemokine).
Biography
Ludger Klein is Professor at the Institute for Immunology of the University of Munich. His group is interested in functional adaptations of thymic stromal cells for T cell repertoire selection. A second research focus is unravelling the parameters that discriminate clonal deletion and TReg differentiation as alternative cell fate choices of autoreactive thymocytes.
Bruno Kyewski is head of the Division of Developmental Immunology at the German Cancer Research Center. His laboratory studies the structure–function relationship of the thymic microenvironment. Current research in his laboratory focuses on three areas: the cellular and molecular mechanisms underlying the phenomenon of promiscuous gene expression by thymic epithelial cells, the developmental biology of thymic epithelial cells and the role of central tolerance in human autoimmune diseases.
Paul Allen is the Robert L. Kroc Professor of Pathology and Immunology at Washington University. His laboratory has had a long-standing interest in the processing and presentation of self-proteins and how self-peptide/MHC complexes play a critical role in positive selection and peripheral T cell function.
Kris Hogquist is Professor and Associate Director of the Center for Immunology at the University of Minnesota. Her group studies how thymic selection processes shape the T cell repertoire. Their current research is focused in three areas: positive and negative selection in the thymus, iNKT cell development, and the human T cell response to EBV.
References
- 1.Kyewski B, Klein L. A central role for central tolerance. Annual review of immunology. 2006;24:571–606. doi: 10.1146/annurev.immunol.23.021704.115601. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa Y, Ohigashi I, Nitta T, Sakata M, Tanaka K, et al. Thymic nurse cells provide microenvironment for secondary T cell receptor alpha rearrangement in cortical thymocytes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20572–20577. doi: 10.1073/pnas.1213069109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nature reviews. Immunology. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 4.Florea BI, Verdoes M, Li N, van der Linden WA, Geurink PP, et al. Activity-based profiling reveals reactivity of the murine thymoproteasome-specific subunit beta5t. Chemistry & biology. 2010;17:795–801. doi: 10.1016/j.chembiol.2010.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata S, Sasaki K, Kishimoto T, Niwa S, Hayashi H, et al. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science. 2007;316:1349–1353. doi: 10.1126/science.1141915. [DOI] [PubMed] [Google Scholar]
- 6.Nakagawa T, Roth W, Wong P, Nelson A, Farr A, et al. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science. 1998;280:450–453. doi: 10.1126/science.280.5362.450. [DOI] [PubMed] [Google Scholar]
- 7.Gommeaux J, Gregoire C, Nguessan P, Richelme M, Malissen M, et al. Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes. European journal of immunology. 2009;39:956–964. doi: 10.1002/eji.200839175. [DOI] [PubMed] [Google Scholar]
- 8.Nedjic J, Aichinger M, Mizushima N, Klein L. Macroautophagy, endogenous MHC II loading and T cell selection: the benefits of breaking the rules. Current opinion in immunology. 2009;21:92–97. doi: 10.1016/j.coi.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 10.Honey K, Nakagawa T, Peters C, Rudensky A. Cathepsin L regulates CD4+ T cell selection independently of its effect on invariant chain: a role in the generation of positively selecting peptide ligands. The Journal of experimental medicine. 2002;195:1349–1358. doi: 10.1084/jem.20011904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitta T, Murata S, Sasaki K, Fujii H, Ripen AM, et al. Thymoproteasome shapes immunocompetent repertoire of CD8+ T cells. Immunity. 2010;32:29–40. doi: 10.1016/j.immuni.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Xing Y, Jameson SC, Hogquist KA. Thymoproteasome subunit-beta5T generates peptide-MHC complexes specialized for positive selection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6979–6984. doi: 10.1073/pnas.1222244110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziegler A, Muller CA, Bockmann RA, Uchanska-Ziegler B. Low-affinity peptides and T-cell selection. Trends in immunology. 2009;30:53–60. doi: 10.1016/j.it.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Ryan KR, McNeil LK, Dao C, Jensen PE, Evavold BD. Modification of peptide interaction with MHC creates TCR partial agonists. Cellular immunology. 2004;227:70–78. doi: 10.1016/j.cellimm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. Journal of Experimental Medicine. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–434. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 17.Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010;32:214–226. doi: 10.1016/j.immuni.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer MJ, Mahajan VS, Chen J, Irvine DJ, Lauffenburger DA. Signaling thresholds govern heterogeneity in IL-7-receptor-mediated responses of naive CD8(+) T cells. Immunology and cell biology. 2011;89:581–594. doi: 10.1038/icb.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013;38:263–274. doi: 10.1016/j.immuni.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4(+) T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014;15:266–274. doi: 10.1038/ni.2822. [Refs. 18 and 19 find that T cell responsiveness is set in the thymus and maintained in mature T cells in proportion to the avidity of the positively selecting interaction. Ref. 18 concludes that T cells with stronger affinity for self dominate in responses to infections, whereas Ref. 19 challenges the generality of such correlations.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372:100–103. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 22.Daley SR, Hu DY, Goodnow CC. Helios marks strongly autoreactive CD4+ T cells in two major waves of thymic deletion distinguished by induction of PD-1 or NF-kappaB. The Journal of experimental medicine. 2013;210:269–285. doi: 10.1084/jem.20121458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stritesky GL, Xing Y, Erickson JR, Kalekar LA, Wang X, et al. Murine thymic selection quantified using a unique method to capture deleted T cells. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4679–4684. doi: 10.1073/pnas.1217532110. [Using different approaches, these two studies quantify ‘early’ and ‘late’ negative selection in the cortex and the medulla, respectively, and conclude that the extent of clonal deletion in the cortex exceeds that in the medulla.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. The Journal of experimental medicine. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melichar HJ, Ross JO, Herzmark P, Hogquist KA, Robey EA. Distinct temporal patterns of T cell receptor signaling during positive versus negative selection in situ. Science signaling. 2013;6:ra92. doi: 10.1126/scisignal.2004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irla M, Hollander G, Reith W. Control of central self-tolerance induction by autoreactive CD4+ thymocytes. Trends in immunology. 2010;31:71–79. doi: 10.1016/j.it.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 27.Mathis D, Benoist C. Aire. Annual review of immunology. 2009;27:287–312. doi: 10.1146/annurev.immunol.25.022106.141532. [DOI] [PubMed] [Google Scholar]
- 28.Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nature reviews. Immunology. 2008;8:948–957. doi: 10.1038/nri2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. Journal of Experimental Medicine. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oukka M, Cohen-Tannoudji M, Tanaka Y, Babinet C, Kosmatopoulos K. Medullary thymic epithelial cells induce tolerance to intracellular proteins. Journal of immunology. 1996;156:968–975. [PubMed] [Google Scholar]
- 31.Hinterberger M, Aichinger M, Prazeres da Costa O, Voehringer D, Hoffmann R, et al. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nature immunology. 2010;11:512–519. doi: 10.1038/ni.1874. [Through diminution of MHC class II on mTECs, this study documents an autonomous contribution of mTECs to both dominant and recessive mechanisms of CD4+ T cell tolerance and provides experimental support for the avidity model of TReg cell development versus clonal deletion.] [DOI] [PubMed] [Google Scholar]
- 32.Klein L, Klein T, Ruther U, Kyewski B. CD4 T cell tolerance to human C-reactive protein, an inducible serum protein, is mediated by medullary thymic epithelium. The Journal of experimental medicine. 1998;188:5–16. doi: 10.1084/jem.188.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oukka M, Colucci-Guyon E, Tran PL, Cohen-Tannoudji M, Babinet C, et al. CD4 T cell tolerance to nuclear proteins induced by medullary thymic epithelium. Immunity. 1996;4:545–553. doi: 10.1016/s1074-7613(00)80481-1. [DOI] [PubMed] [Google Scholar]
- 34.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature immunology. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 35.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. Journal of immunology. 2009;183:7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klein L, Roettinger B, Kyewski B. Sampling of complementing self-antigen pools by thymic stromal cells maximizes the scope of central T cell tolerance. European journal of immunology. 2001;31:2476–2486. doi: 10.1002/1521-4141(200108)31:8<2476::aid-immu2476>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 37.Munz C. Enhancing immunity through autophagy. Annual review of immunology. 2009;27:423–449. doi: 10.1146/annurev.immunol.021908.132537. [DOI] [PubMed] [Google Scholar]
- 38.Aichinger M, Wu C, Nedjic J, Klein L. Macroautophagy substrates are loaded onto MHC class II of medullary thymic epithelial cells for central tolerance. The Journal of experimental medicine. 2013;210:287–300. doi: 10.1084/jem.20122149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizushima N. Autophagy in protein and organelle turnover. Cold Spring Harbor symposia on quantitative biology. 2011;76:397–402. doi: 10.1101/sqb.2011.76.011023. [DOI] [PubMed] [Google Scholar]
- 40.Dongre AR, Kovats S, deRoos P, McCormack AL, Nakagawa T, et al. In vivo MHC class II presentation of cytosolic proteins revealed by rapid automated tandem mass spectrometry and functional analyses. European journal of immunology. 2001;31:1485–1494. doi: 10.1002/1521-4141(200105)31:5<1485::AID-IMMU1485>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 41.Klein L, Hinterberger M, von Rohrscheidt J, Aichinger M. Autonomous versus dendritic cell-dependent contributions of medullary thymic epithelial cells to central tolerance. Trends in immunology. 2011;32:188–193. doi: 10.1016/j.it.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 42.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. The Journal of experimental medicine. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 44.Taniguchi RT, DeVoss JJ, Moon JJ, Sidney J, Sette A, et al. Detection of an autoreactive T-cell population within the polyclonal repertoire that undergoes distinct autoimmune regulator (Aire)-mediated selection. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:7847–7852. doi: 10.1073/pnas.1120607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, et al. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 46.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. The Journal of experimental medicine. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fan Y, Rudert WA, Grupillo M, He J, Sisino G, et al. Thymus-specific deletion of insulin induces autoimmune diabetes. The EMBO journal. 2009;28:2812–2824. doi: 10.1038/emboj.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehrlich LI, Oh DY, Weissman IL, Lewis RS. Differential contribution of chemotaxis and substrate restriction to segregation of immature and mature thymocytes. Immunity. 2009;31:986–998. doi: 10.1016/j.immuni.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Borgne M, Ladi E, Dzhagalov I, Herzmark P, Liao YF, et al. The impact of negative selection on thymocyte migration in the medulla. Nature immunology. 2009;10:823–830. doi: 10.1038/ni.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ueda Y, Katagiri K, Tomiyama T, Yasuda K, Habiro K, et al. Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus. Nature communications. 2012;3:1098. doi: 10.1038/ncomms2105. [DOI] [PubMed] [Google Scholar]
- 51.Klein L. Dead man walking: how thymocytes scan the medulla. Nature immunology. 2009;10:809–811. doi: 10.1038/ni0809-809. [DOI] [PubMed] [Google Scholar]
- 52.Derbinski J, Pinto S, Rosch S, Hexel K, Kyewski B. Promiscuous gene expression patterns in single medullary thymic epithelial cells argue for a stochastic mechanism. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:657–662. doi: 10.1073/pnas.0707486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinto S, Michel C, Schmidt-Glenewinkel H, Harder N, Rohr K, et al. Overlapping gene coexpression patterns in human medullary thymic epithelial cells generate self-antigen diversity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3497–3505. doi: 10.1073/pnas.1308311110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villasenor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15854–15859. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Seminars in immunology. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. The Journal of experimental medicine. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nature reviews. Immunology. 2012;12:557–569. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 58.Proietto AI, Lahoud MH, Wu L. Distinct functional capacities of mouse thymic and splenic dendritic cell populations (vol 86, pg 700, 2007). Immunology and Cell Biology. 2009;87:190–190. doi: 10.1038/icb.2008.63. [DOI] [PubMed] [Google Scholar]
- 59.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. The Journal of experimental medicine. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. Journal of immunology. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 61.Atibalentja DF, Murphy KM, Unanue ER. Functional redundancy between thymic CD8alpha+ and Sirpalpha+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. Journal of immunology. 2011;186:1421–1431. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baba T, Badr Mel S, Tomaru U, Ishizu A, Mukaida N. Novel process of intrathymic tumor-immune tolerance through CCR2-mediated recruitment of Sirpalpha+ dendritic cells: a murine model. PloS one. 2012;7:e41154. doi: 10.1371/journal.pone.0041154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reizis B, Colonna M, Trinchieri G, Barrat F, Gilliet M. Plasmacytoid dendritic cells: one-trick ponies or workhorses of the immune system? Nature reviews. Immunology. 2011;11:558–565. doi: 10.1038/nri3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Wirnsberger G, Mair F, Klein L. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:10278–10283. doi: 10.1073/pnas.0901877106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, et al. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [This study shows that endogenous pDCs take up subcutaneously injected antigen and transport it to the thymus in a CCR9-dependent fashion. Upon intravenous injection, antigen-loaded pDCs deleted specific thymocytes, revealing that migratory pDCs can support central tolerance.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, et al. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nature immunology. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, et al. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nature immunology. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 69.Akashi K, Richie LI, Miyamoto T, Carr WH, Weissman IL. B lymphopoiesis in the thymus. Journal of immunology. 2000;164:5221–5226. doi: 10.4049/jimmunol.164.10.5221. [DOI] [PubMed] [Google Scholar]
- 70.Feyerabend TB, Terszowski G, Tietz A, Blum C, Luche H, et al. Deletion of Notch1 converts pro-T cells to dendritic cells and promotes thymic B cells by cell-extrinsic and cell-intrinsic mechanisms. Immunity. 2009;30:67–79. doi: 10.1016/j.immuni.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 71.Mori S, Inaba M, Sugihara A, Taketani S, Doi H, et al. Presence of B cell progenitors in the thymus. Journal of immunology. 1997;158:4193–4199. [PubMed] [Google Scholar]
- 72.Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:17011–17016. doi: 10.1073/pnas.1313001110. [Using B cell receptor (BCR) and TCR transgenic mice, this study shows that autoreactive thymic B cells serve as efficient APCs for negative selection. Thymic B cells may capture autoantigens through their BCR and present these to developing thymocytes for clonal deletion.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frommer F, Waisman A. B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PloS one. 2010;5:e15372. doi: 10.1371/journal.pone.0015372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kleindienst P, Chretien I, Winkler T, Brocker T. Functional comparison of thymic B cells and dendritic cells in vivo. Blood. 2000;95:2610–2616. [PubMed] [Google Scholar]
- 75.Guerri L, Peguillet I, Geraldo Y, Nabti S, Premel V, et al. Analysis of APC types involved in CD4 tolerance and regulatory T cell generation using reaggregated thymic organ cultures. Journal of immunology. 2013;190:2102–2110. doi: 10.4049/jimmunol.1202883. [DOI] [PubMed] [Google Scholar]
- 76.Yuseff MI, Pierobon P, Reversat A, Lennon-Dumenil AM. How B cells capture, process and present antigens: a crucial role for cell polarity. Nature reviews. Immunology. 2013;13:475–486. doi: 10.1038/nri3469. [DOI] [PubMed] [Google Scholar]
- 77.Weiss S, Bogen B. MHC class II-restricted presentation of intracellular antigen. Cell. 1991;64:767–776. doi: 10.1016/0092-8674(91)90506-t. [DOI] [PubMed] [Google Scholar]
- 78.Munthe LA, Corthay A, Os A, Zangani M, Bogen B. Systemic autoimmune disease caused by autoreactive B cells that receive chronic help from Ig V region-specific T cells. Journal of immunology. 2005;175:2391–2400. doi: 10.4049/jimmunol.175.4.2391. [DOI] [PubMed] [Google Scholar]
- 79.Detanico T, Heiser RA, Aviszus K, Bonorino C, Wysocki LJ. Self-tolerance checkpoints in CD4 T cells specific for a peptide derived from the B cell antigen receptor. Journal of immunology. 2011;187:82–91. doi: 10.4049/jimmunol.1002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nature immunology. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, et al. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nature immunology. 2009;10:1155–1161. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martin B, Auffray C, Delpoux A, Pommier A, Durand A, et al. Highly self-reactive naive CD4 T cells are prone to differentiate into regulatory T cells. Nature communications. 2013;4:2209. doi: 10.1038/ncomms3209. [DOI] [PubMed] [Google Scholar]
- 83.Hsieh CS, Lee HM, Lio CW. Selection of regulatory T cells in the thymus. Nature reviews. Immunology. 2012;12:157–167. doi: 10.1038/nri3155. [DOI] [PubMed] [Google Scholar]
- 84.Wirnsberger G, Hinterberger M, Klein L. Regulatory T-cell differentiation versus clonal deletion of autoreactive thymocytes. Immunology and cell biology. 2011;89:45–53. doi: 10.1038/icb.2010.123. [DOI] [PubMed] [Google Scholar]
- 85.Cowan JE, Parnell SM, Nakamura K, Caamano JH, Lane PJ, et al. The thymic medulla is required for Foxp3+ regulatory but not conventional CD4+ thymocyte development. The Journal of experimental medicine. 2013;210:675–681. doi: 10.1084/jem.20122070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klein L, Jovanovic K. Regulatory T cell lineage commitment in the thymus. Seminars in immunology. 2011;23:401–409. doi: 10.1016/j.smim.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 87.Mathis D, Benoist C. A decade of AIRE. Nature reviews. Immunology. 2007;7:645–650. doi: 10.1038/nri2136. [DOI] [PubMed] [Google Scholar]
- 88.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, et al. Aire-dependent thymic development of tumor-associated regulatory T cells. Science. 2013;339:1219–1224. doi: 10.1126/science.1233913. [This study reports that TReg cells recurrently enriched in prostate tumors of mice recognized an unknown antigen that was also present in the healthy prostate. These cells were found to differentiate as ‘natural’ (i.e. thymically induced) TReg cells in an Aire-dependent manner, providing evidence for a link between Aire-mediated expression of peripheral tissue antigens and the development of organ-specific TReg cells.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, et al. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nature immunology. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leung MW, Shen S, Lafaille JJ. TCR-dependent differentiation of thymic Foxp3+ cells is limited to small clonal sizes. The Journal of experimental medicine. 2009;206:2121–2130. doi: 10.1084/jem.20091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. The Journal of experimental medicine. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.St-Pierre C, Brochu S, Vanegas JR, Dumont-Lagace M, Lemieux S, et al. Transcriptome sequencing of neonatal thymic epithelial cells. Scientific reports. 2013;3:1860. doi: 10.1038/srep01860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lv H, Havari E, Pinto S, Gottumukkala RV, Cornivelli L, et al. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. The Journal of clinical investigation. 2011;121:1561–1573. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gottumukkala RV, Lv H, Cornivelli L, Wagers AJ, Kwong RY, et al. Myocardial infarction triggers chronic cardiac autoimmunity in type 1 diabetes. Science translational medicine. 2012;4:138ra180. doi: 10.1126/scitranslmed.3003551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. The Journal of experimental medicine. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Durinovic-Bello I, Wu RP, Gersuk VH, Sanda S, Shilling HG, et al. Insulin gene VNTR genotype associates with frequency and phenotype of the autoimmune response to proinsulin. Genes and immunity. 2010;11:188–193. doi: 10.1038/gene.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pugliese A, Zeller M, Fernandez A, Jr., Zalcberg LJ, Bartlett RJ, et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nature genetics. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- 98.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nature genetics. 1997;15:289–292. doi: 10.1038/ng0397-289. [DOI] [PubMed] [Google Scholar]
- 99.Giraud M, Taubert R, Vandiedonck C, Ke X, Levi-Strauss M, et al. An IRF8-binding promoter variant and AIRE control CHRNA1 promiscuous expression in thymus. Nature. 2007;448:934–937. doi: 10.1038/nature06066. [DOI] [PubMed] [Google Scholar]
- 100.Colobran R, Armengol Mdel P, Faner R, Gartner M, Tykocinski LO, et al. Association of an SNP with intrathymic transcription of TSHR and Graves’ disease: a role for defective thymic tolerance. Human molecular genetics. 2011;20:3415–3423. doi: 10.1093/hmg/ddr247. [DOI] [PubMed] [Google Scholar]
- 101.Klein L, Klugmann M, Nave KA, Tuohy VK, Kyewski B. Shaping of the autoreactive T-cell repertoire by a splice variant of self protein expressed in thymic epithelial cells. Nature medicine. 2000;6:56–61. doi: 10.1038/71540. [DOI] [PubMed] [Google Scholar]
- 102.de Jong VM, Abreu JR, Verrijn Stuart AA, van der Slik AR, Verhaeghen K, et al. Alternative splicing and differential expression of the islet autoantigen IGRP between pancreas and thymus contributes to immunogenicity of pancreatic islets but not diabetogenicity in humans. Diabetologia. 2013;56:2651–2658. doi: 10.1007/s00125-013-3034-6. [DOI] [PubMed] [Google Scholar]
- 103.Scally SW, Petersen J, Law SC, Dudek NL, Nel HJ, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. The Journal of experimental medicine. 2013 doi: 10.1084/jem.20131241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.van Lummel M, Duinkerken G, van Veelen PA, de Ru A, Cordfunke R, et al. Post-Translational Modification Of Hla-Dq Binding Islet-Autoantigens In Type 1 Diabetes. Diabetes. 2013 doi: 10.2337/db12-1214. [DOI] [PubMed] [Google Scholar]
- 105.Gascoigne NR, Palmer E. Signaling in thymic selection. Current opinion in immunology. 2011;23:207–212. doi: 10.1016/j.coi.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bains I, van Santen HM, Seddon B, Yates AJ. Models of self-peptide sampling by developing T cells identify candidate mechanisms of thymic selection. PLoS Comput Biol. 2013;9:e1003102. doi: 10.1371/journal.pcbi.1003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Org T, Chignola F, Hetenyi C, Gaetani M, Rebane A, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO reports. 2008;9:370–376. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Koh AS, Kuo AJ, Park SY, Cheung P, Abramson J, et al. Aire employs a histone-binding module to mediate immunological tolerance, linking chromatin regulation with organ-specific autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15878–15883. doi: 10.1073/pnas.0808470105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abramson J, Giraud M, Benoist C, Mathis D. Aire's partners in the molecular control of immunological tolerance. Cell. 2010;140:123–135. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 110.Giraud M, Yoshida H, Abramson J, Rahl PB, Young RA, et al. Aire unleashes stalled RNA polymerase to induce ectopic gene expression in thymic epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:535–540. doi: 10.1073/pnas.1119351109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Danso-Abeam D, Humblet-Baron S, Dooley J, Liston A. Models of aire-dependent gene regulation for thymic negative selection. Frontiers in immunology. 2011;2:14. doi: 10.3389/fimmu.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Marrack P, Ignatowicz L, Kappler JW, Boymel J, Freed JH. Comparison of peptides bound to spleen and thymus class II. The Journal of experimental medicine. 1993;178:2173–2183. doi: 10.1084/jem.178.6.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Collado JA, Alvarez I, Ciudad MT, Espinosa G, Canals F, et al. Composition of the HLA-DR-associated human thymus peptidome. European journal of immunology. 2013 doi: 10.1002/eji.201243280. [DOI] [PubMed] [Google Scholar]
- 114.Espinosa G, Collado JA, Scholz E, Mestre-Ferrer A, Kuse N, et al. Peptides presented by HLA class I molecules in the human thymus. Journal of proteomics. 2013;94C:23–36. doi: 10.1016/j.jprot.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 115.Adamopoulou E, Tenzer S, Hillen N, Klug P, Rota IA, et al. Exploring the MHC-peptide matrix of central tolerance in the human thymus. Nature communications. 2013;4:2039. doi: 10.1038/ncomms3039. [DOI] [PubMed] [Google Scholar]
- 116.Fortier MH, Caron E, Hardy MP, Voisin G, Lemieux S, et al. The MHC class I peptide repertoire is molded by the transcriptome. The Journal of experimental medicine. 2008;205:595–610. doi: 10.1084/jem.20071985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mester G, Hoffmann V, Stevanovic S. Insights into MHC class I antigen processing gained from large-scale analysis of class I ligands. Cellular and molecular life sciences : CMLS. 2011;68:1521–1532. doi: 10.1007/s00018-011-0659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Millet V, Naquet P, Guinamard RR. Intercellular MHC transfer between thymic epithelial and dendritic cells. European journal of immunology. 2008;38:1257–1263. doi: 10.1002/eji.200737982. [DOI] [PubMed] [Google Scholar]