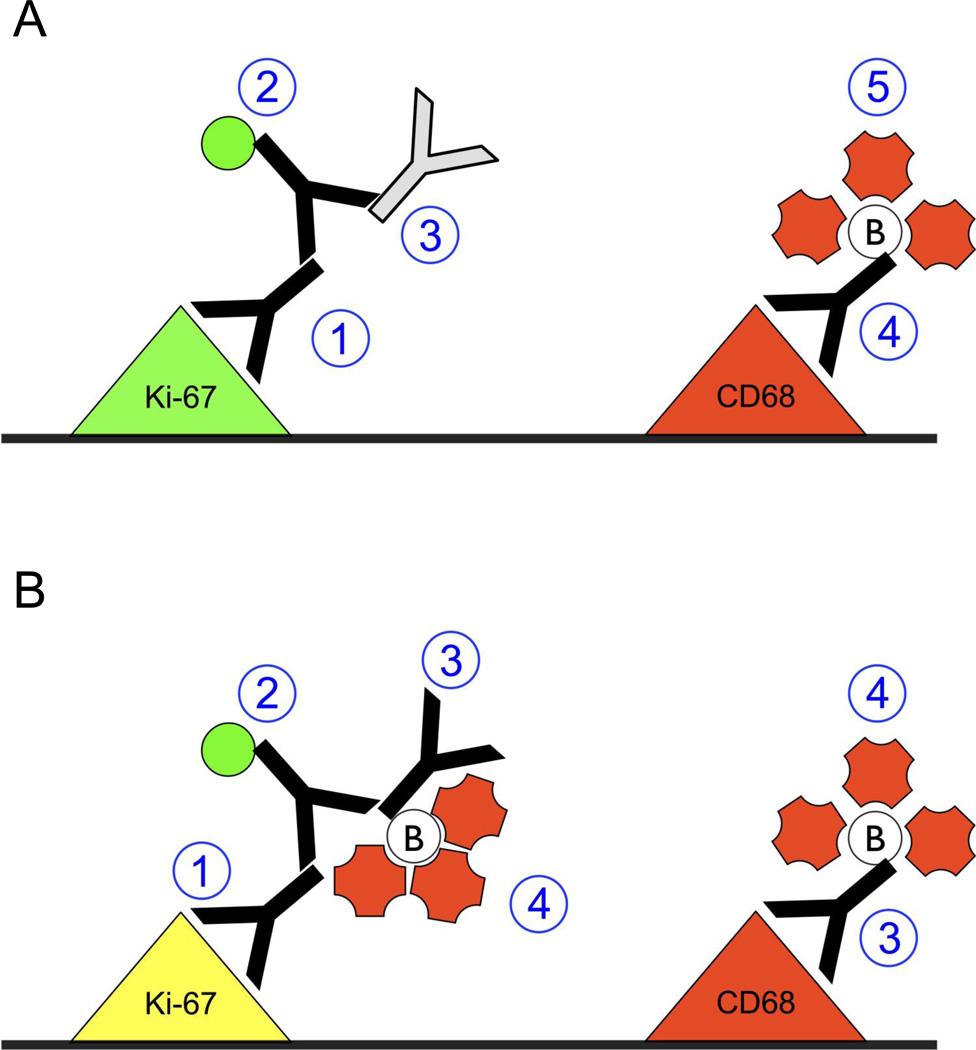
Fig. 6.
Diagrammatic representation of the steps employed for performing double immunofluorescent staining with two mouse IgG1 antibodies, with each step enumerated with blue circles. a Illustration of staining protocol in which an unconjugated isotype control antibody is used to prevent interactions between tissue-bound anti-mouse secondary antibody and the biotinylated mouse antibody. First, the unconjugated primary antibody is added to the tissue to detect the first protein of interest. Second, the conjugated secondary antibody is added to the tissue to detect the unconjugated primary antibody. Third, an unconjugated isotype control is added to saturate the free Fab fragments on tissue-bound anti-mouse secondary antibody. Fourth, biotinylated primary antibody is added to the tissue detect the second protein of interest. Fifth, conjugated streptavidin is added to the tissue to detect the biotinylated primary antibody. See Online Resource 1 for an animated version of this diagram. b Illustration of staining protocol that results in artifactual colocalization (as seen in Fig. 5) caused by interactions between tissue-bound anti-mouse secondary antibody and the biotinylated mouse antibody. First, the unconjugated primary antibody is added to the tissue to detect the first protein of interest. Second, the conjugated secondary antibody is added to the tissue to detect the unconjugated primary antibody. Third, the biotinylated primary antibody is added to the tissue, where it not only binds to its target protein but also to the free arms on the secondary antibody that is bound to the tissue. Fourth, conjugated streptavidin is added to detect the biotinylated primary antibody, resulting in red fluorescence in iNOS-positive areas as well as in CD68-positive areas. See Online Resource 2 for an animated version of this diagram