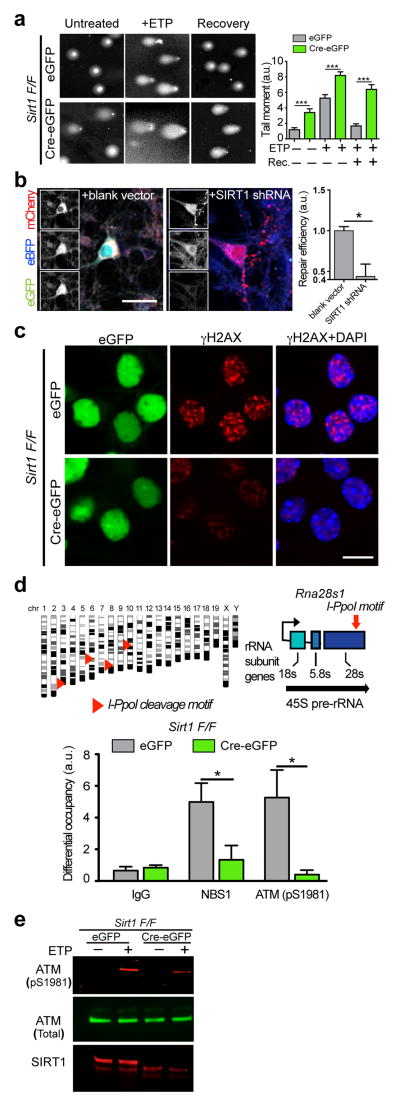
Figure 1. SIRT1 is necessary for initial DSB signaling events and DNA repair in neurons.
a, Sirt1 F/F neurons were infected with lentiviral vectors carrying either a functional Cre recombinase (Cre-eGFP) or a non-functional Cre (eGFP) were treated with 5μM etoposide for 1h, and were either allowed to recover for 16h in the absence of etoposide or lysed immediately. DNA damage was then assessed using the comet assay. Graph indicates “comet tail moments” (***p< 0.001, n = at least 50 per condition, one-way ANOVA). b, Cultured primary neurons were transfected with a pre-digested NHEJ reporter construct (see also Supplementary Figs. 1b and 1c) together with either scrambled shRNA or SIRT1 shRNA and the number of GFP+ cells were assessed to indicate NHEJ-mediated repair (* p < 0.05, unpaired t-test). c, Sirt1 F/F neurons infected as in a were treated with either vehicle or 2μM etoposide, following which the cells were fixed and stained with antibodies to γH2AX. d, A synthetic, inducible system encoding the rare-cutting homing endonuclease, I-PpoI, was used to generate DSBs at defined regions within the genomes of primary Sirt1 F/F neurons transduced as in a. I-PpoI cleavage sites in the Rna28s1 locus are depicted (top). Results from ChIP experiments measuring the recruitment of ATM (pS1981) and NBS1 to cleavage sites within the Rna28s1 locus (bottom) (*p < 0.05, student’s t-test). e, Sirt1 F/F neurons infected as in a were treated with either vehicle or 5μM etoposide, and levels of phosphorylated ATM were compared by western blotting.