Abstract
Background
Leukaemia is the eleventh commonest UK cancer. The four main subtypes have different clinical profiles, particularly between chronic and acute types.
Aim
To identify the symptom profiles of chronic and acute leukaemia in adults in primary care.
Design and setting
Matched case-control studies using Clinical Practice Research Datalink records.
Method
Putative symptoms of leukaemia were identified in the year before diagnosis. Conditional logistic regression was used for analysis, and positive predictive values (PPVs) were calculated to estimate risk.
Results
Of cases diagnosed between 2000 and 2009, 4655 were aged ≥40 years (2877 chronic leukaemia (CL), 937 acute leukaemia (AL), 841 unreported subtype). Ten symptoms were independently associated with CL, the three strongest being: lymphadenopathy (odds ratio [OR] 22, 95% confidence interval [CI] = 13 to 36), weight loss (OR 3.0, 95% CI = 2.1 to 4.2), and bruising (OR 2.3, 95% CI = 1.6 to 3.2). Thirteen symptoms were independently associated with AL, the three strongest being: nosebleeds and/or bleeding gums (OR 5.7, 95% CI = 3.1 to 10), fever (OR 5.3, 95% CI = 2.7 to 10), and fatigue (OR 4.4, 95% CI = 3.3 to 6.0). No individual symptom or combination of symptoms had a PPV >1%.
Conclusion
The symptom profiles of CL and AL have both overlapping and distinct features. This presents a dichotomy for GPs: diagnosis, by performing a full blood count, is easy; however, the symptoms of leukaemia are non-specific and of relatively low risk. This explains why many leukaemia diagnoses are unexpected findings.
Keywords: acute lymphoblastic leukaemia, acute myeloid leukaemia, chronic leukaemia, diagnosis, primary health care
INTRODUCTION
Leukaemia is characterised by proliferation of abnormal leukocytes.1 There are four main subtypes: chronic myeloid leukaemia (CML), chronic lymphocytic leukaemia (CLL), acute myeloid leukaemia (AML), and acute lymphoblastic leukaemia (ALL). The incidence, clinical presentation, and survival all vary by subtype. Around 8600 people in the UK are diagnosed with leukaemia, with 4800 deaths annually. It is mainly a disease of adults, with 88% of new diagnoses occurring in those aged >40 years.1–5
The latest leukaemia survival figures show a reduction in the percentage of ‘avoidable UK deaths’ from 4.5% (1985– 1989) to 1.3% (1995–1999); however, a discrepancy in mortality between the rich and poor still exists in England.6,7 Updated UK guidance on the diagnosis of leukaemia gives several symptoms that GPs may consider investigating by a full blood count; however, none of these recommendations was based on primary care evidence.8
Diagnosis generally follows symptomatic presentation to a health professional, usually in primary care, prompting blood testing or through an incidental blood test result when leukaemia had not been considered. No primary care symptomatic articles have been published. One secondary care article reported the percentage of patients with symptomatic presentations: these were least common in CLL (47%) compared with 71% in CML, 77% in AML, and 78% in ALL. Tiredness and pain were common to all leukaemias but some symptoms were more specific: chest pain in acute leukaemia (AL), bruising/bleeding and shortness of breath/cough in AML, masses in ALL and CLL, and abnormal sweating in chronic leukaemia (CL).9 Another study of over 5000 CLL patients reported infection in 32%, fatigue in 17%, and lymphadenopathy in 7%, with splenomegaly and excessive sweating being much rarer.10 For CML, fatigue has been reported in nearly 34%, with bleeding and weight loss in 21% and 20%, respectively.11 Infection, headache, cough, malaise, and nausea were rarer, reported in 7% of cases. For the acute leukaemias, fatigue, haemorrhage, fever, and infection are common in AML, whereas fatigue, infection, bone pain, fever, bruising/bleeding, and petechiae have been reported in ALL.12,13
Haematological abnormalities in CLL have been reported up to 10 years before diagnosis, with monoclonal B-cell lymphocytosis (MBL) believed to be a precursor condition in almost all CLL patients.14 Declining haemoglobin values have been reported in lymphatic leukaemias beginning 5 years before diagnosis.15
This study aimed at identifying and quantifying the separate primary care symptom-only profiles of chronic and acute leukaemias, to guide GPs on when to initiate blood tests for possible leukaemia. Lymphocyte count changes in the 5 years before a diagnosis of CLL were also assessed.
How this fits in
Leukaemia can be diagnosed in primary care through blood tests, either incidentally or once the disease is considered. The differences between the chronic (CL) and acute leukaemia (AL) prodromes are not clear, however. Individual and combined symptom risk estimates are all <1%, making guidance for GPs as to when to take blood tests difficult. The lymphocyte count increases greatly in chronic lymphocytic leukaemia cases in the 6 months before diagnosis, although some patients have raised counts for several years before diagnosis.
METHOD
This was a matched case-control study using electronic patient record data from the UK’s Clinical Practice Research Datalink (CPRD). The methods follow those of previous primary care cancer articles.16,17 The CPRD contains anonymised primary care medical records from around 680 UK general practices, representing 8.8% of the population. Personal information is collected, as well as clinical events such as symptom reporting, diagnoses, prescriptions, and investigation results.
Cases and controls
A list of 96 leukaemia diagnostic codes (available from the authors) was used to identify cases in the CPRD. Cases were aged ≥40 years, diagnosed with leukaemia between 2000 and 2009 inclusive. For each case, up to five age-, sex-, and general practice-matched controls were chosen. The first leukaemia code was taken as the date of diagnosis, or ‘index date’. Controls matched their cases’ index date. Cases were categorised to ‘chronic leukaemia’ (CL), ‘acute leukaemia’ (AL), or ‘undetermined’ based on their first code subtype. Forty cases (33 CL and seven AL) had additional multiple codes for leukaemia, including five with codes for both AL and CL on their index date: all five were assigned to CL, after examining subsequent disease codes. Exclusion criteria were: cases with reticulo-endothelial cancer or thrombocytic leukaemia and their matched controls; any case or control with <1 year of records before the index date; cases without controls; controls with leukaemia; and controls who had not sought medical care after registration.
Selection of putative clinical variables
A list of reported clinical features of leukaemia from research literature and cancer websites was compiled and supplemented with self-reported symptoms from an online patient support group. PubMed, EBSCO, and Google Scholar were used with the search terms ‘acute/chronic leukaemia symptoms’, ‘chronic/acute leukaemia reported to GP’, and ‘early signs/indications/symptoms of acute/chronic leukaemia’. The CPRD contains many codes associated with each feature. A symptom library was therefore compiled for each clinical feature, and their occurrences identified in the year before the index date. Only those features present in ≥2% of cases entered analysis.
Blood test results were not included in the primary analysis as most patients having blood tests will have a full blood count, which almost always reveals any leukaemia. Secondary analyses included adding the white cell count to the final multivariable model, and examined pre-diagnostic lymphocyte counts over 5 years. Recording bias was tested using a feature thought to have no association with leukaemia: fracture.
Analysis and statistical methods
The main analytical method was conditional logistic regression. Variables with a P-value ≤0.1 from univariable analysis entered multivariable analysis. Features were grouped according to similarity; such as abdominal pain, back pain, and chest pain. All features from the group stage attaining a P-value threshold ≤0.05 entered final modelling, which used a P≤0.01 threshold for retention. Clinically plausible interaction terms and lymphoid/myeloid interactions were tested against the final model and retained if their P-value was ≤0.01.
Positive predictive values (PPVs) for the risk of CL or AL were calculated separately using Bayes’ theorem (prior odds × likelihood ratio = posterior odds). Prior odds were calculated from the age-specific national incidence of CL and AL for 2008, expressed as odds. PPVs were estimated for consulting patients only: thus, the posterior odds for CL were divided by 0.910 as 1272 (9%) of 14 103 eligible controls were non-consulters. The conversion factor for AL was 0.915. A separate analysis of the lymphocyte count (selected in preference to the white cell count [WCC], as it is more specific for CLL) was conducted in CLL, and values in the 5 years before diagnosis plotted as a monthly moving average.
Power calculation
Initial estimates from the CPRD indicated that approximately 3000 CL and 1000 AL cases were available; these transpired to be minor overestimates. A power calculation showed that for CL there was >98% power to detect a difference in a rare variable present in 2% cases and 1% of controls. For a more common variable, there was >99% power to detect a difference in prevalence of 20% in cases to 15% in controls. For AL, power was >98% for detecting a difference from 3% in cases to 1% in controls, and >96% power for detecting a difference from 20% in cases and 15% in controls. All analysis was conducted using Stata software (version 13.1).
RESULTS
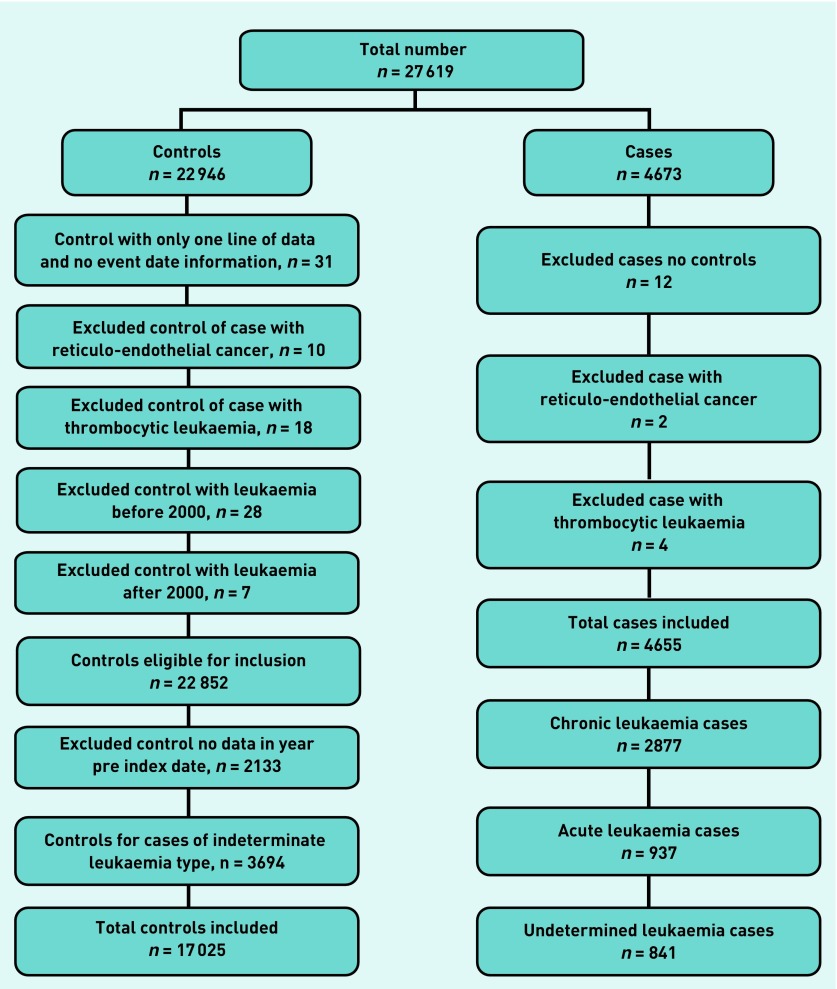
The CPRD supplied 27 619 patients (4673 cases and 22 946 controls). After applying the exclusion criteria (Figure 1), 4655 cases and 22 852 controls remained. There were 2877 CL cases and 12 811 controls, and 937 AL cases and 4214 controls. The remainder were undetermined leukaemia subtypes, and these were omitted.
Figure 1.
Leukaemia exclusions.
Patient demographic and consultation information is given in Table 1. CL and AL cases consulted significantly more often than controls in the year before diagnosis (P ≤0.001; rank-sum test).
Table 1.
Patient demographics and consultation rates in the year before diagnosis
| Chronic leukaemia | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases | Controls | |||||
|
|
|
|||||
| Male (n = 1679) | Female (n = 1198) | Total (n = 2877) | Male (n = 7281) | Female (n = 5530) | Total (n = 12 811) | |
| Median age at diagnosis, years (IQR) | 71 (62–79) | 74 (64–81) | 72 (63–80) | 71 (63–79) | 73 (65–81) | 72 (64–80) |
| Median number of consultations (IQR) | 12 (7–20) | 13 (8–20) | 13a (8–20) | 8 (4–14) | 9 (4–15) | 8a (4–15) |
| Acute leukaemia | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Cases | Controls | |||||
|
|
|
|||||
| Male (n = 541) | Female (n = 3962) | Total (n = 937) | Male (n = 2377) | Female (n = 1837) | Total (n = 4214) | |
| Median age at diagnosis, years (IQR) | 72 (63–78) | 73 (64–81) | 72 (63–80) | 72 (65–78) | 73 (64–81) | 73 (64–80) |
| Median number of consultations (IQR) | 16 (9–24) | 16 (9–25) | 16a (9–25) | 8 (4–15) | 9 (4–16) | 8a (4–16) |
Cases consulted significantly more often than controls in the year before diagnosis (P ≤0.001). IQR = interquartile range.
Symptoms
Fifty symptoms were considered initially. Ten remained significant in the CL final model; 13 in AL. Their frequencies, univariable likelihood ratios, and multivariable odds ratios (ORs) are shown in Table 2. From the 2877 CL cases, 1400 (48.7%) had either no symptoms or symptoms not present in the final model, compared with 351 (37.5%) of the 937 AL cases. Some previously reported symptoms were too uncommon for reliable analysis: bone pain, 1.7% in CL, 1.5% in AL; splenomegaly, 0.9% CL, 0.5% AL; excessive sweating, 0.8% CL, 0.7% AL; and petechiae, 0.6% CL, 0.7% AL. Within CL, there was a significant interaction of lymphoid versus myeloid leukaemia and lymphadenopathy (general and site-specific), with lymphadenopathy being reported more in CLL cases (interaction OR 0.014, P <0.001). For AL, there was a significant sex interaction for chest pain, with more males reporting the symptom (OR 0.403, P <0.005). The proportion of patients with a fracture in both CL and AL did not differ between cases and controls (P <0.33 and P <0.62, respectively).
Table 2.
Symptoms of acute and chronic leukaemia
| Symptom | Acute leukaemia | |||
|---|---|---|---|---|
|
| ||||
| Cases, n (%) n = 937 | Controls, n (%) n = 4214 | Likelihood ratio, (95% CI) | OR in multivariable analysis (95% CI) | |
| Infectiona | 237 (25) | 651 (15) | 1.6 (1.4 to 1.9) | 1.5 (1.3 to 1.8) |
| Shortness of breath | 136 (15) | 248 (6) | 2.5 (2.0 to 3.0) | 2.5 (1.9 to 3.2) |
| Fatigue | 109 (12) | 128 (3) | 3.8 (3.0 to 4.9) | 4.4 (3.3 to 6.0) |
| Chest pain | 90 (10) | 202 (5) | 2.0 (1.6 to 2.5) | 1.5 (1.1 to 2.1) |
| Abdominal pain | 89 (10) | 202 (5) | 2.0 (1.6 to 2.5) | 1.7 (1.2 to 2.2) |
| Diarrhoea | 64 (7) | 118 (3) | 2.4 (1.8 to 3.3) | 2.2 (1.5 to 3.1) |
| Malaise | 57 (6) | 58 (1) | 4.4 (3.1 to 6.3) | 3.4 (2.2 to 5.2) |
| Vomiting and nausea | 56 (6) | 98 (2) | 2.6 (1.9 to 3.5) | 1.8 (1.2 to 2.6) |
| Bruisingb | 41 (4) | 52 (1) | 3.6 (2.4 to 5.3) | 3.7 (2.3 to 5.8) |
| Fever | 28 (3) | 19 (0.5) | 6.6 (3.7 to 12) | 5.3 (2.7 to 10) |
| Nosebleeds and bleeding gums | 26 (3) | 30 (0.7) | 3.9 (2.3 to 6.6) | 5.7 (3.1 to 10) |
| Flu | 20 (2) | 24 (0.6) | 3.8 (2.1 to 6.8) | 3.9 (2.0 to 7.5) |
| Weight loss | 18 (2) | 33 (1) | 2.5 (1.4 to 4.3) | 3.0 (1.5 to 5.8) |
| Symptom | Chronic leukaemia | |||
|---|---|---|---|---|
|
| ||||
| Cases, n (%) n = 2877 | Controls, n (%) n = 12 811 | Likelihood ratio, (95% CI) | OR in multivariable analysis (95% CI) | |
| Infectiona | 608 (21) | 1887 (15) | 1.4 (1.3 to 1.6) | 1.5 (1.3 to 1.6) |
| Cough | 415 (14) | 1394 (11) | 1.3 (1.2 to 1.5) | 1.2 (1.1 to 1.4) |
| Hypertension | 403 (14) | 1619 (13) | 1.1 (1.0 to 1.2) | 1.2 (1.1 to 1.4) |
| Shortness of breath | 214 (7) | 666 (5) | 1.4 (1.2 to 1.7) | 1.3 (1.1 to 1.5) |
| Fatigue | 196 (7) | 409 (3) | 2.1 (1.8 to 2.5) | 2.1 (1.8 to 2.6) |
| Diarrhoea | 138 (5) | 381 (3) | 1.6 (1.3 to 2.0) | 1.4 (1.1 to 1.7) |
| Lymphadenopathy | 90 (3) | 18 (0.1) | 22 (13 to 37) | 22 (13 to 36) |
| Malaise | 70 (2) | 170 (1) | 1.8 (1.4 to 2.4) | 1.7 (1.3 to 2.3) |
| Weight loss | 65 (2) | 93 (1) | 3.1 (2.3 to 4.3) | 3.0 (2.1 to 4.2) |
| Bruisingb | 58 (2) | 115 (1) | 2.3 (1.6 to 3.1) | 2.3 (1.6 to 3.2) |
Infection consists of urinary tract infection, upper respiratory tract infection, skin infection, and chest infection symptoms.
Bruising consists of bruising, haematoma, and contusion symptoms.
The secondary analysis of the CL dataset incorporating raised WCCs was very different, containing only five variables, and dominated by the very high OR of the raised WCC: raised WCC OR 81 (95% confidence interval [CI] = 64 to 102), lymphadenopathy OR 21 (95% CI = 11 to 41), bruising/haematoma/contusion OR 1.9 (95% CI = 1.3 to 3.0), fatigue OR 1.6 (95% CI = 1.3 to 2.1), and infection OR 1.4 (95% CI = 1.2 to 1.6).
Positive predictive values
PPVs were calculated for those aged >60 years, targeting patients around the average age of leukaemia diagnosis; accounting for 83% of CL cases and 82% of AL cases. For CL, the highest PPV was 0.34% for lymphadenopathy; weight loss, bruising, and fatigue were all <0.01%. Lymphadenopathy with cough had the highest combined PPV of 0.27%; many combinations were too rare to allow a PPV calculation. For AL, all single symptoms had PPVs <0.01%. Fever with infection produced the highest combined PPV of 0.13%.
Lymphocyte counts in the years before diagnosis of CLL
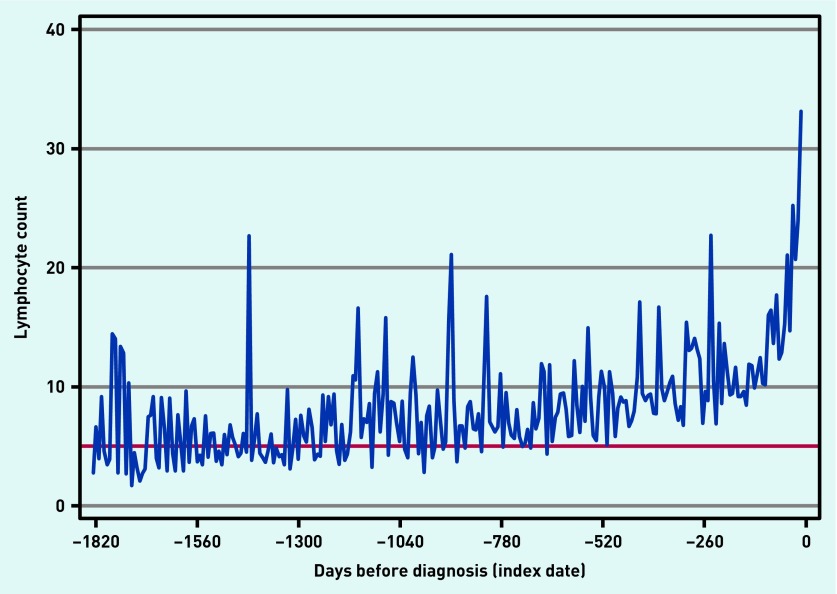
The lymphocyte counts in 1751 out of 2379 (73.6%) CLL cases in the 5 years before the index date are shown in Figure 2. The graph indicates a steady increase in lymphocyte count in cases, becoming greater in the 6 months before a CLL diagnosis. Many abnormally high values were present up to 5 years before diagnosis.
Figure 2.
Lymphocyte count in patients with chronic lymphocytic leukaemia up to 5 years before their diagnosis presented as a monthly moving average. Note: the red horizontal line represents a lymphocyte count of 5000 (per cubic millimetre), the threshold value at which CLL is diagnosed.18
DISCUSSION
Summary
This is the first study to investigate separate CL and AL symptoms from primary care. Ten were independently associated with CL; 13 with AL. Non-specific symptoms such as fatigue, infection, malaise, weight loss, diarrhoea, bruising, and shortness of breath were common to both CL and AL. The remaining symptoms were unique to the subtype: lymphadenopathy, cough, and hypertension were significant in CL, whereas nosebleeds and bleeding gums, fever, flu, abdominal pain, chest pain, and vomiting and nausea were significant in AL. No single symptom or symptom pair had a PPV of >1%. The raised lymphocyte count in CLL cases up to 5 years before diagnosis suggests that the diagnosis could be advanced considerably, although the clinical advantages of this are debatable.
Leukaemia is relatively rare. A number of symptoms were identified related to either chronic or acute leukaemia — or both. No symptom stands out as a high-risk marker of the disease, although many of the symptoms would lead to testing, which would then reveal the underlying leukaemia. This probably explains why most diagnoses are serendipitous, and are likely to remain so, especially as the number of patients having blood counts increases.
Strengths and limitations
The use of the CPRD ensured good data quality, a large sample size, and a representative spread of patients across the UK. Analysis by subtype, sex, and age was possible, allowing the identification of relevant symptoms, although even this large dataset was insufficient for some symptom combinations, which were simply too rare in controls. The selection of putative symptoms was inclusive and derived from literature review, supplemented by self-reported symptoms. Pertinent symptoms probably were not excluded.
Nearly one-fifth of the total leukaemia cases had a generic leukaemia code that could not be categorised to either AL or CL: these cases were omitted from the study. This is unlikely to have introduced bias, although it will have reduced power a little.
Recording of duration and severity of symptoms is generally poor in GP notes, and GPs appear to record diagnoses preferentially over symptoms. Information on cancer staging is also poor, preventing sub-analyses of, for example, early-stage cancers. GPs can choose to record information in a free text (‘uncoded’) section, which is generally not available to researchers. This can influence the results if such differential recording occurs preferentially in either cases or controls — although this affect appears to be minor.19 Cases have a higher attendance rate, thereby increasing the chance of reporting a symptom. However, a test for this bias of the fracture rate did not support this potential concern.
Comparison with existing literature
Tiredness has been reported in the secondary care literature: both malaise and fatigue were present, but with a higher prevalence in AL.9–11 Similarly, chest pain was found to be associated with AL only.9 Bruising, previously reported in AML patients, was found in both CL and AL, but twice as often in AL. Shortness of breath, also previously reported in patients with AML, was significant in both CL and AL; cough was found only in CL, as has been reported for CML previously.11 The symptom with the highest OR, lymphadenopathy, which was previously reported in ALL and CLL, was found only in CLL patients in this study.9 Hypertension was significantly associated with CL, but with such a small OR as to be of little value clinically. Finally, raised lymphocytes long before a CLL diagnosis has been reported previously in the secondary care literature.14
Implications for research and practice
In theory, primary care diagnosis of leukaemia should be easy, as nearly all leukaemias have abnormal full blood counts. Indeed, many leukaemias, especially CLL, are identified serendipitously, with possible leukaemia not considered likely — or at all — at the time of testing.9 Ideally, this study would have separated the truly asymptomatic group of patients from those with a symptom not included in the final model; however, this was not possible, as the methods included only putative leukaemia symptoms, and omitted symptoms deemed irrelevant. Most patients having a full blood count were probably symptomatic, even if the symptoms were not those of leukaemia. Only 51% of the CL patients had a symptom appearing in the final model; this suggests that roughly half are serendipitously discovered. Thus, the present study results should be seen as symptoms that prompt consideration of a blood count. The symptoms largely match those in the revised National Institute for Health and Care Excellence (NICE) guidance, which combined all forms of leukaemia. Shortness of breath, chest and abdominal pain, and diarrhoea are not included in the guidance, however.
It was not clear what events had triggered full blood counts in the study cases, particularly those who were apparently asymptomatic. Blood testing is remarkably common in primary care, with approximately one-quarter of those aged >30 years having a full blood count in any 1 year.20
The clinical problem is that all PPVs are tiny. All the symptoms reported here have much more likely benign alternative explanations, although some of the alternative diagnoses, such as anaemia, would require a blood count to be taken for diagnosis. In truth, however, the small possibility of leukaemia will add only marginally to the decision to investigate by a blood count. Some symptoms, such as fatigue, are investigated frequently, particularly when persistent.21 A second group of symptoms often suggests important underlying disease, such as weight loss, bruising, nosebleeds, bleeding gums, or lymphadenopathy, especially in those aged >60 years. The present results suggest that a blood count should be included in investigation of these. The graph of lymphocyte counts before diagnosis of CLL (Figure 2) suggests that there is a considerable opportunity to expedite the diagnosis; this could include software in the primary care clinical systems or in the reporting laboratory, which could alert the GP to a raised WCC, highlighting the possibility of CLL. This is supported by the analysis retaining WCCs in the chronic leukaemia model, showing a very high OR with only lymphadenopathy adding much to the predictive power of the model. Indeed, it is plausible other haematological measures are changing in the years before CLL is diagnosed. This is an avenue for further study. Although treatment of early CLL is sometimes considered, many patients are observed without active treatment initially, so the imperative to diagnose early is arguably much less in CLL than in most other cancers.
Acknowledgments
The authors would like to acknowledge the contribution to the research presented in this article made by the Discovery Programme Steering Committee comprising: Roger Jones (chair); Jonathan Banks; Alison Clutterbuck; Jon Emery; Joanne Hartland; Sandra Hollinghurst; Maire Justice; Jenny Knowles; Helen Morris; Tim Peters; Greg Rubin.
Funding
This article presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Grant Reference Number RP-PG-0608– 10045). Fiona M Walter is part-funded by an NIHR Clinician Scientist award. Richard D Neal is part-funded by Public Health Wales and Betsi Cadwaladr University Health Board. Willie Hamilton is supported by the NIHR Collaboration for Leadership in Applied Health Research and Care South West Peninsula at the Royal Devon and Exeter NHS Foundation Trust. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Ethical approval
Independent Scientific Advisory Committee (protocol 09–110).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Willie Hamilton is clinical lead on the ongoing revision of the NICE guidance on investigation of suspected cancer. His contribution to this article is in a personal capacity, and is not to be interpreted as representing the view of the Guideline Development Group, or of NICE itself. Peter W Rose reports personal fees from GP Update Ltd, outside the submitted work. The other authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Cancer Research UK Cancer statistics report: cancer incidence and mortality in the UK, September 2014. 2014. http://publications.cancerresearchuk.org/downloads/Product/CS_REPORT_TOP10INCMORT.pdf (accessed 15 Jan 2016).
- 2.Information Services Division. Cancer incidence in Scotland, 2013. Secondary cancer incidence in Scotland, 2013. 2015 http://www.isdscotland.org/Health-Topics/Cancer/Publications/data-tables.asp?id=1387#1387 (accessed 15 Jan 2016). [Google Scholar]
- 3.Northern Ireland Cancer Registry Leukaemia: incidence and survival 1993–2013. Secondary leukaemia: incidence and survival 1993–2013. 2015. http://www.qub.ac.uk/research-centres/nicr/CancerInformation/official-statistics/BySite/Blood/ (accessed 15 Jan 2016).
- 4.Office for National Statistics Cancer statistics registrations, England. Secondary cancer statistics registrations, England. 2015 http://www.ons.gov.uk/ons/rel/vsob1/cancer-statistics-registrations--england--series-mb1-/no--44--2013/index.html (accessed 15 Jan 2016). [Google Scholar]
- 5.Welsh Cancer Intelligence and Surveillance Unit Cancer in Wales Secondary cancer in Wales. 2015 http://www.wcisu.wales.nhs.uk/cancer-statistics (accessed 15 Jan 2016). [Google Scholar]
- 6.Abdel-Rahman M, Stockton D, Rachet B, et al. What if cancer survival in Britain were the same as in Europe: how many deaths are avoidable? Br J Cancer. 2009;101(S2):S115–S124. doi: 10.1038/sj.bjc.6605401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis L, Coleman MP, Rachet B. How many deaths would be avoidable if socioeconomic inequalities in cancer survival in England were eliminated? A national population-based study, 1996–2006. Eur J Cancer. 2012;48(2):270–278. doi: 10.1016/j.ejca.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence . Suspected cancer: recognition and referral NG12. London: NICE; 2015. http://www.nice.org.uk/guidance/NG12 (accessed 15 Jan 2016). [PubMed] [Google Scholar]
- 9.Howell DA, Smith AG, Jack A, et al. Time-to-diagnosis and symptoms of myeloma, lymphomas and leukaemias: a report from the Haematological Malignancy Research Network. BMC Hematol. 2013;13(1):9. doi: 10.1186/2052-1839-13-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friese CR, Earle CC, Magazu LS, et al. Timeliness and quality of diagnostic care for medicare recipients with chronic lymphocytic leukemia. Cancer. 2011;117(7):1470–1477. doi: 10.1002/cncr.25655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Savage DG, Szydlo RM, Goldman JM. Clinical features at diagnosis in 430 patients with chronic myeloid leukaemia seen at a referral centre over a 16-year period. Br J Haematol. 1997;96(1):111–116. doi: 10.1046/j.1365-2141.1997.d01-1982.x. [DOI] [PubMed] [Google Scholar]
- 12.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. New Engl J Med. 1999;341(14):1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 13.University of Maryland Medical Center Acute lymphocytic leukemia. 2013. https://umm.edu/health/medical/reports/articles/acute-lymphocytic-leukemia (accessed 15 Jan 2016).
- 14.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113(22):5412–5417. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edgren G, Bagnardi V, Bellocco R, et al. Pattern of declining hemoglobin concentration before cancer diagnosis. Int J Cancer. 2010;127(6):1429–1436. doi: 10.1002/ijc.25122. [DOI] [PubMed] [Google Scholar]
- 16.Shephard E, Neal R, Rose P, et al. Clinical features of kidney cancer in primary care: a case-control study using primary care records. Br J Gen Pract. 2013 doi: 10.3399/bjgp13X665215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stapley S, Peters TJ, Neal RD, et al. The risk of oesophago-gastric cancer in symptomatic patients in primary care: a large case-control study using electronic records. Br J Cancer. 2013;108(1):25–31. doi: 10.1038/bjc.2012.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawstron AC, Bennett FL, O’Connor SJM, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–583. doi: 10.1056/NEJMoa075290. [DOI] [PubMed] [Google Scholar]
- 19.Price S, Shephard E, Stapley S, et al. Non-visible versus visible haematuria and bladder cancer risk: a study of electronic records in primary care. Br J Gen Pract. 2014 doi: 10.3399/bjgp14X681409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton W, Lancashire R, Sharp D, et al. The importance of anaemia in diagnosing colorectal cancer: a case-control study using electronic primary care records. Br J Cancer. 2008;98(2):323–327. doi: 10.1038/sj.bjc.6604165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamilton W, Watson J, Round A. Investigating fatigue in primary care. BMJ. 2010;341:c4259. doi: 10.1136/bmj.c4259. [DOI] [PubMed] [Google Scholar]