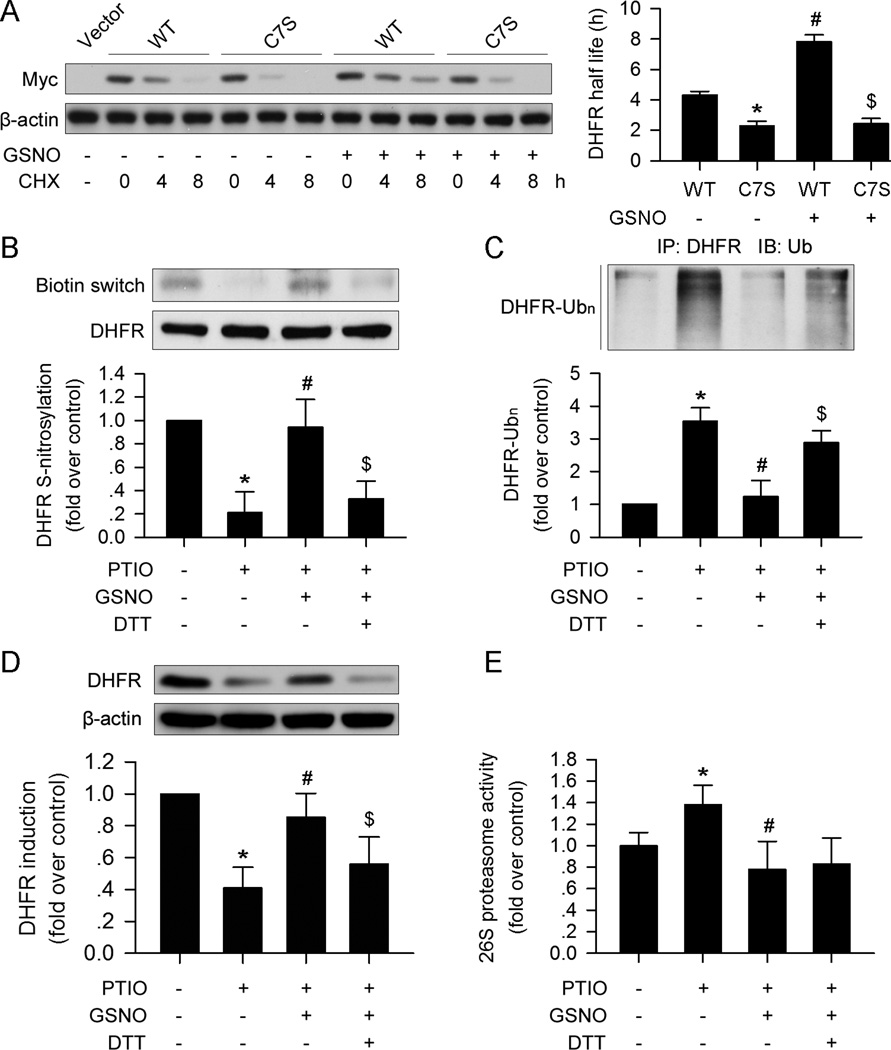
Figure 4. DHFR S-nitrosylation prevents ubiquitination and degradation.
(A) The C7S mutation in DHFR destabilized the protein compared with the wild type DHFR treated with CHX (30µg/ml). GSNO (100µM) stabilized DHFR, while the C7S mutation abolished the effect. (B) GSNO (100µM) restored DHFR S-nitrosylation, which was suppressed by PTIO (150µM). DTT (10mM) inhibited the effect of GSNO (100µM). (C and D) GSNO (100µM) prevented DHFR ubiquitination and degradation induced by PTIO (150µM); this effect could be blocked by DTT (10mM) supplementation. (E) PTIO (150µM) promoted 26S proteasome activity, which could be reversed by GSNO (100µM). The addition of DTT (10mM) did not affect 26S proteasome activity compared with the PTIO+GSNO group (n=3; *p<0.05 vs. WT in A, or p<0.05 vs. control in B–E; #p<0.05 vs. C7S in A, or p<0.05 vs. PTIO in B–E; $p<0.05 vs. PTIO+GSNO in B–D).