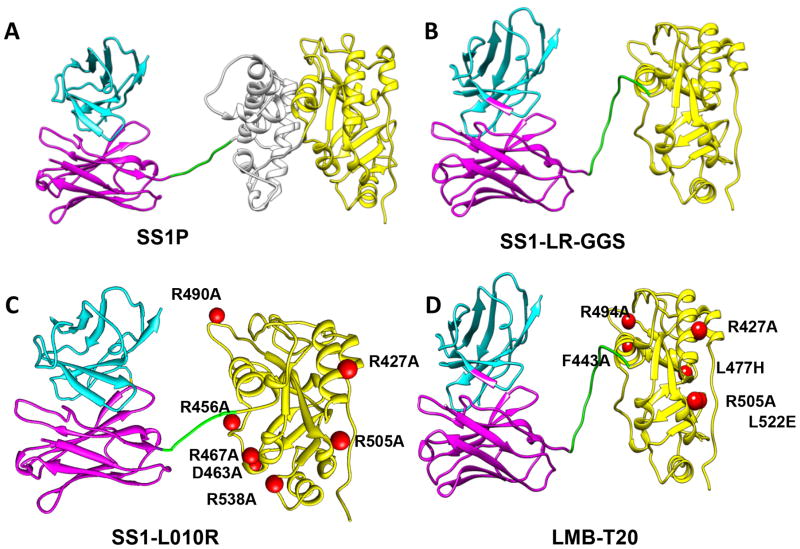
Figure 1. Structural models of RITs.
The RIT SS1P consists of the disulfide-stabilized VH and VL polypeptide chains of the Fv from the antimesothelin monoclonal antibody SS1 coupled to a 38-kDa fragment of PE38. (A) SS1P. The Fv (cyan and magenta) is recombinantly connected to PE38, which is divided into domain II (gray), domain III (yellow), and part of domain Ib from native PE38. (B) SS1-LR-GGS. Deletion of domain II with GGS linker between the linker and the domain III. (C) SS1-LO10R. PE24 with six point mutations in domain III designed to eliminate binding to B cell receptor. (D) LMB-T20. PE24 with six point mutations in domain III designed to diminish T cell epitopes. All models are hypothetical arrangements based on the structures of native PE and immunoglobulin G; they do not represent actual structure determinations