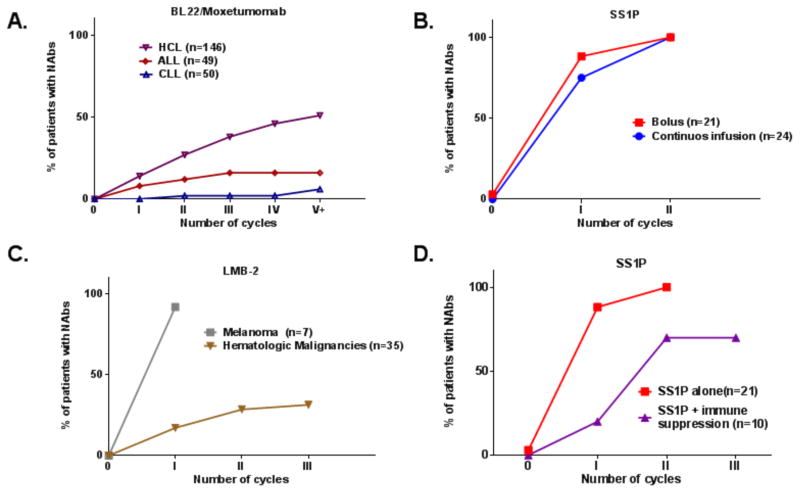
Figure 2. Neutralizing antibody formation for PE38 RITs.
Summary of immunogenicity rate during treatment cycles from nine clinical trials. Immunogenicity rate was evaluated using functional Nab assay with a cut point of 75% neutralization in all trials (17, 19, 22, 27, 28, 38) and unpublished data. (A) HCL (n=146), ALL (n=49) and CLL patients (n=50) treated with Moxetumomab paseudotox or BL22. (B) Patients treated with SS1P by bolus (QOD x 3) (n=21) or continues infusion (n=24) over the course of 10 days. (C) Immunogenicity of LMB-2 in hematological (n=35) and melanoma patients (n=7). (D) Immunogenicity in patients treated with SS1P as a monotherapy (n=21) or in SS1P combined with Pentostatin and Cyclophosphamide (n=11). All patients (with the exaptation of continues infusion group) were treated in a similar schedule of 3x QOD bolus per cycle, and 21-day intervals between cycles.