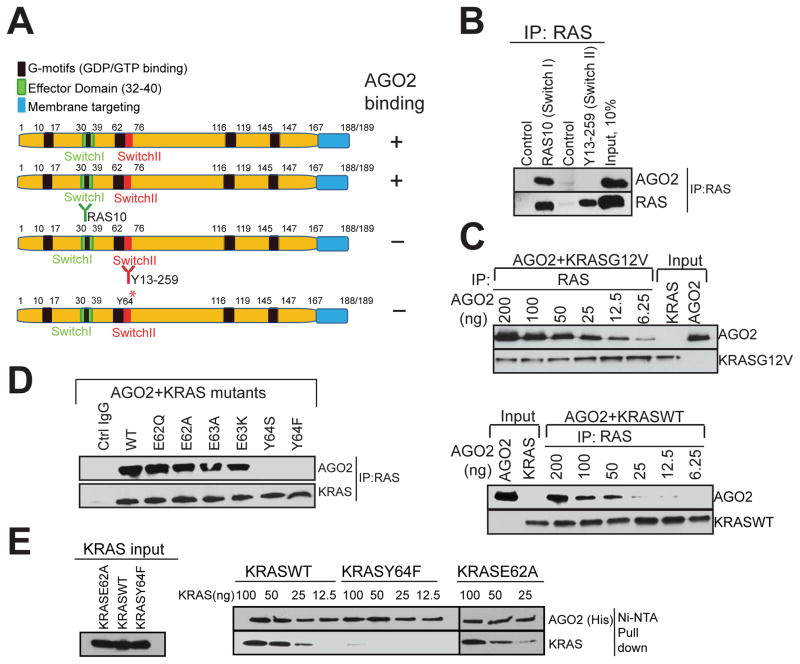
Figure 4. The Switch II domain of RAS interacts with AGO2.
(A) Schematic summary of the antibodies and recombinant proteins used for RAS-AGO2 co-IP analysis to identify residues in RAS, critical for AGO2 interaction. (B) RAS co-IP using antibodies that bind switch I domain (RAS10 Ab) or switch II domain (Y13-259 Ab), followed by immunoblot analysis for RAS and AGO2. (C–E) Characterization of direct RAS-AGO2 interaction, in vitro. (C) Immunoblot analysis following in vitro co-IP of recombinant KRASG12V (top panel) and KRASWT (bottom panel) in the presence of varying concentrations of recombinant AGO2. (D) In vitro co-IP analysis of KRAS-AGO2 interaction using a panel of KRAS mutant proteins spanning amino acid residues 62–65 in the switch II domain. (E) Immunoblot analysis following His-AGO2 pull down assay using Ni-NTA beads upon incubation with different KRAS mutant proteins. See also Figure S4.