Figure 6. SIRT2 Deacetylation of ATRIP at K32 Promotes Binding to RPA-ssDNA.
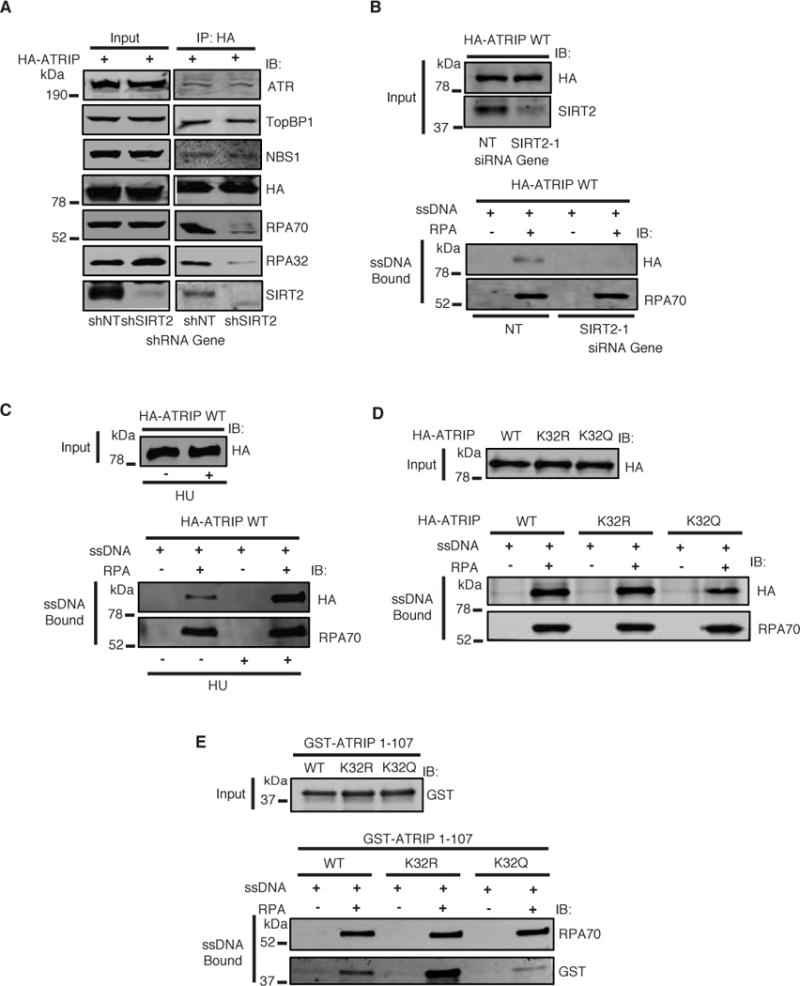
(A) HeLa cells stably expressing SIRT2 or control shRNA were transfected with HA-ATRIP and treated with HU in the presence of TSA, harvested, immunoprecipitated with anti-HA beads, separated by SDS-PAGE, and immunoblotted with antibodies against ATR, TopBP1, NBS1, HA, RPA70, RPA32 and SIRT2. (B–D) HA-ATRIP expressed in 293T cells was incubated with biotinylated ssDNA bound to streptavidin beads with or without recombinant RPA for 2 hours. Bound proteins were washed, separated by SDS-PAGE, and immunoblotted with the indicated antibodies. (B) RPA-ssDNA binding assay for HA-ATRIP WT expressed in 293T cells and transfected with SIRT2 or control siRNA. (C) RPA-ssDNA binding assay for HA-ATRIP WT purified from 293T cells treated with or without HU. (D) RPA-ssDNA binding assay for HA-ATRIP WT, K32R, or K32Q purified from 293T cells. (E) Recombinant GST-ATRIP 1–107 WT, K32R, or K32Q was incubated with biotinylated ssDNA bound to streptavidin beads with or without recombinant RPA for 2 hours. Bound proteins were washed, separated by SDS-PAGE, and immunoblotted with the indicated antibodies. See also Figure S6.
