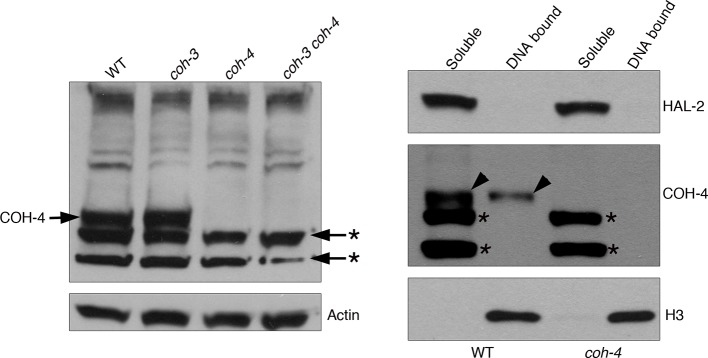
Figure 5. WAPL-1 antagonizes binding of COH-3/4 cohesin to axial elements.
(A) Projections of pachytene nuclei stained with the indicated antibodies and DAPI. In all cases, WT and wapl-1 examples were acquired with the same exposure settings and images are non-deconvolved projections adjusted with same settings to allow visual comparisons in staining intensity. The intensities of SMC-3 and COH-3/4 are increased in wapl-1 mutants, while REC-8 staining appears similar in WT and wapl-1 nuclei. Scale bar = 5 µm. (B) Quantification of mean fluorescence intensity per nucleus of REC-8, COH-3/4 and SMC-3 in transition zone (TZ), early pachytene (EP) and late pachytene (LP) nuclei. Between 15 and 20 nuclei per germ line, from a minimum of 5 germ lines were analyzed per genotype and stage. Differences indicated with asterisks are significant (p<0.0001, t-test), error bars= SEM. (C) Line profile quantification to compare the intensity of cohesin at axial elements versus inter-chromosome domains. Top left-hand side panel: example of a SMC-3 and DAPI-stained pachytene nucleus showing the intensities of DAPI (blue) and SMC-3 (green) along the depicted line. The line profile of SMC-3 intensity is shown in the top right-hand panel, ΔF indicates the increment in staining between the peak (axial element) and the valley (inter chromosome domain as determined by lack of DAPI staining). Graph: Plotting of individual ΔF values from late pachytene nuclei of wapl-1 mutants and WT stained with REC-8, COH-3/4 or SMC-3 antibodies. Between 12 and 32 nuclei from different germ lines were analyzed per genotype. Mean value and SEM are indicated. Proportional increase between the mean ΔF value in wapl-1 and WT: 27% for REC-8 (p= 0.0008, t-test), 114% for COH-3/4 (p<0.0001, t-test), 59% for SMC-3 (p<0.0001, t-test). (D) Western blots of triton soluble and insoluble (DNA bound) protein fractions from WT and wapl-1 mutant worms probed with anti-COH-4 (see Figure 5—figure supplement 5 for additional controls), anti-HAL-2 (marker for soluble fraction), and anti-H3 (marker for DNA-bound fraction) antibodies. Asterisk indicates a non-specific band recognized by anti-COH-4 antibodies (see Figure 5—figure supplement 5). Note that COH-4 signal in WT extracts is higher in the soluble than in the DNA fraction, while in wapl-1 mutant extracts COH-4 intensity is higher in DNA-bound than in the soluble fraction. (E) Quantification of relative intensity of anti-COH-4 signal in the soluble and DNA-bound fractions. Three westerns were included in the analysis.
Figure 5—figure supplement 1. Controls showing specificity of anti-COH-3/4 antibodies.