FIGURE 1.
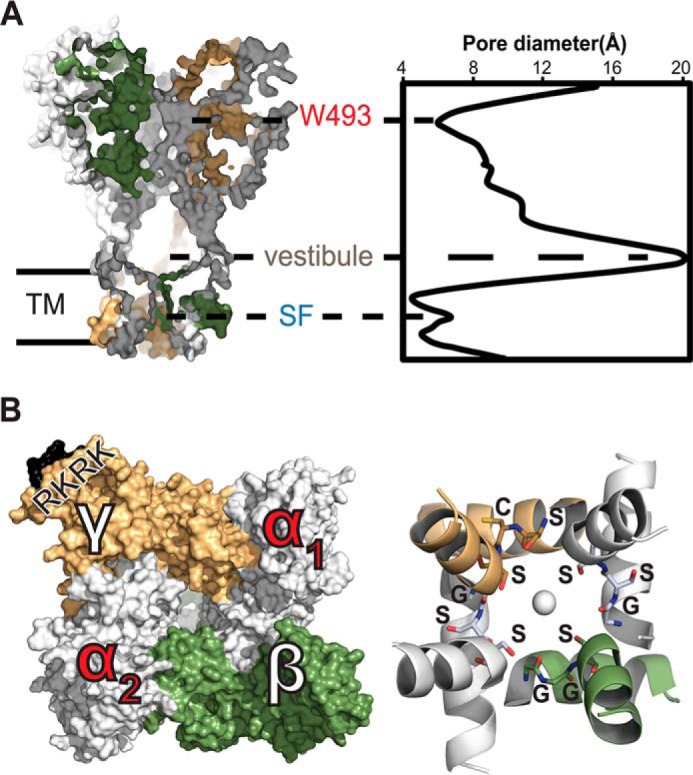
Heterotetramer α1βα2γ model of ENaC. A, left, side view, surface-rendering representation of intersubunit topology. Right, pore geometry of the channel calculated by HOLE (60). Constrictions along the pore axis are labeled at Trp-493, an extracellular site of gain-of-function, and the selectivity filter (SF) in the transmembrane helical bundle. Extracellular vestibule along the pore spans the widest region in the channel. B, left, top view, extracellular surface of intersubunit interfaces. The trypsin cleavage site is colored black and labeled RKRK on the γ-subunit. Right, bottom view, schematic representation of the helical bundle formed by the transmembrane domain TM2 based on the open state of cASIC (Protein Data Bank code 4NTY). The selectivity filter residues αGSS, βGGS, and γSCS are labeled on the structure and shown as sticks. A serine quartet surrounds a hypothetical sodium ion on the channel pore axis.
