FIGURE 6.
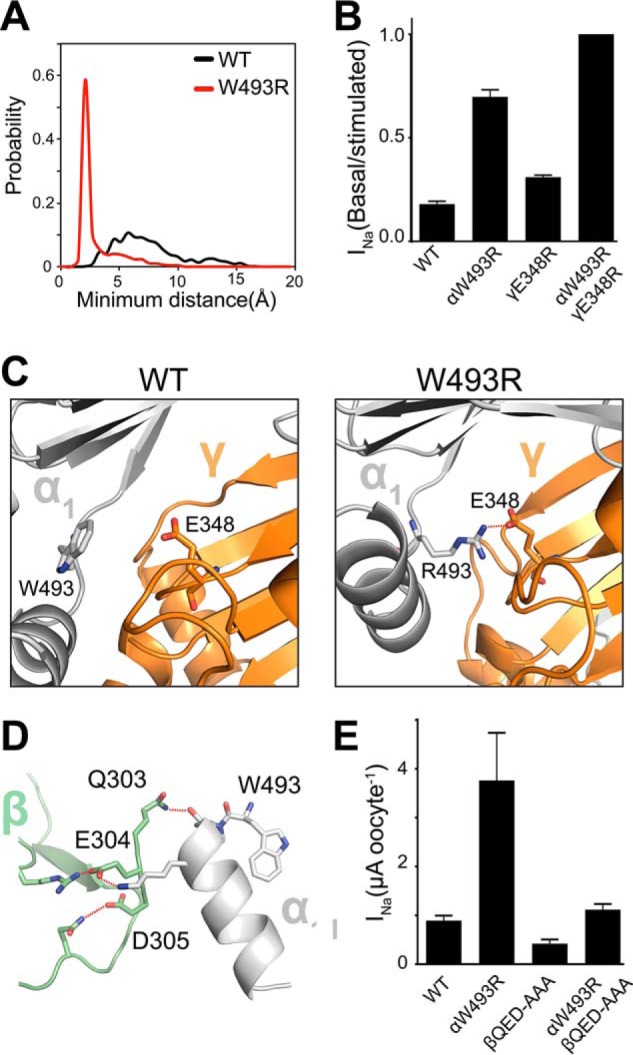
W493R rewires intersubunit interactions. A, DMD simulations indicate an electrostatic interaction between α2Arg-493 (red) and γGlu-348 that is absent in the WT α2-subunit (black). Inter-residue interactions are quantified by a histogram of minimum distance between α2Arg-493 and γGlu-348. B, ENaC currents measured in oocytes injected with cRNA of WT and mutant ENaC subunits; basal current is normalized to trypsin-stimulated current to reflect basal activation. Perturbing the electrostatic interaction by the double mutant γE348R/αW493R channels shows no measurable population of near-silent channels, shown by lack of activation by trypsinization (n = 18, p < 0.0001). C, schematic representation of snapshots from DMD simulations at the α2γ interface. Trp-493/Arg-493 in the α2-subunit (light gray) and Glu-348 in the γ-subunit (orange) are shown as sticks, and hydrogen bonding between γGlu-348 and α2Arg-493 is shown by red dashed lines. D, hydrogen bond network at the α1β intersubunit interface by the acidic patch 303QED305 in the β-subunit with the knuckle domain in the α1-subunit. E, amiloride-sensitive current measured in oocytes. Neutralizing the acidic patch by alanine substitutions decreases W493R activation by ∼2-fold (n = 12, p = 0.002). β303AAA305 slightly inhibits WT ENaC (n = 12, p = 0.008).
