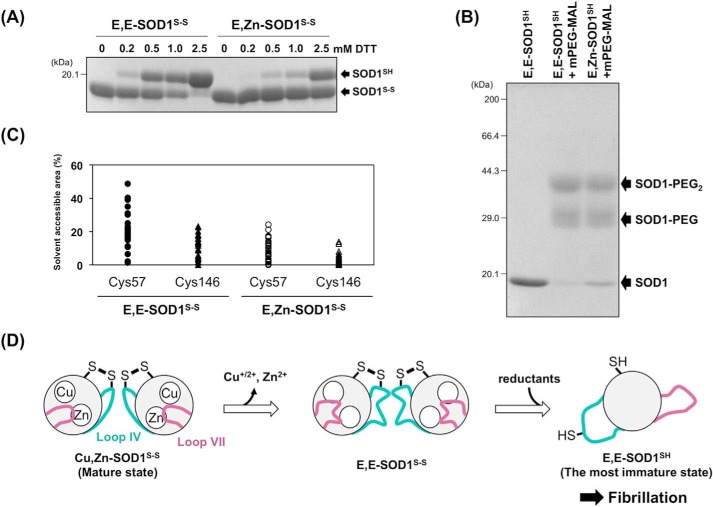
FIGURE 7.
A proposed mechanism of SOD1 fibrillation triggered with conformational disorder of loops IV and VII. A, E,E-SOD1(57/146)S-S and E,Zn-SOD1(57/146)S-S (20 μm) were incubated with the indicated concentrations of dithiothreitol (DTT) at 20 °C for 1 h. To quench the reduction reaction, the proteins were precipitated with trichloroacetic acid and reacted with a thiol-specific modifier, iodoacetamide, in the presence of 2% SDS. SOD1 proteins with and without the disulfide bond were then separated by non-reducing SDS-PAGE. B, E,E-SOD1(57/146)SH and E,Zn-SOD1(57/146)SH (20 μm) were reacted with mPEG-MAL (0.1 mm, Nanocs Inc.) at 10 °C for 1 h. The modification reaction was quenched by adding 10 mm DTT, and the proteins were analyzed with reducing SDS-PAGE. C, solvent-accessible areas of Cys-57 and Cys-146 in apo- and Zn2+-bound SOD1 with a disulfide bond. Each model in the NMR structures of E,Zn-SOD1S-S (35 models: PDB ID, 1KMG) and E,E-SOD1S-S (30 models: PDB ID, 1RK7) was used for calculation of the accessible areas of Cys residues using GetArea. The areas are expressed as a percentage ratio of the surface area in proteins to that in the random coil state. D, a proposed scheme of SOD1 fibrillation. Dissociation of metal ions resulted in significant disorder of loops IV and VII, which allows intracellular reductants to attack the disulfide bond and thereby facilitate the formation of the most immature SOD1. The most immature SOD1 had a unique ellipsoidal conformation, which is accessible to further fibrillation.