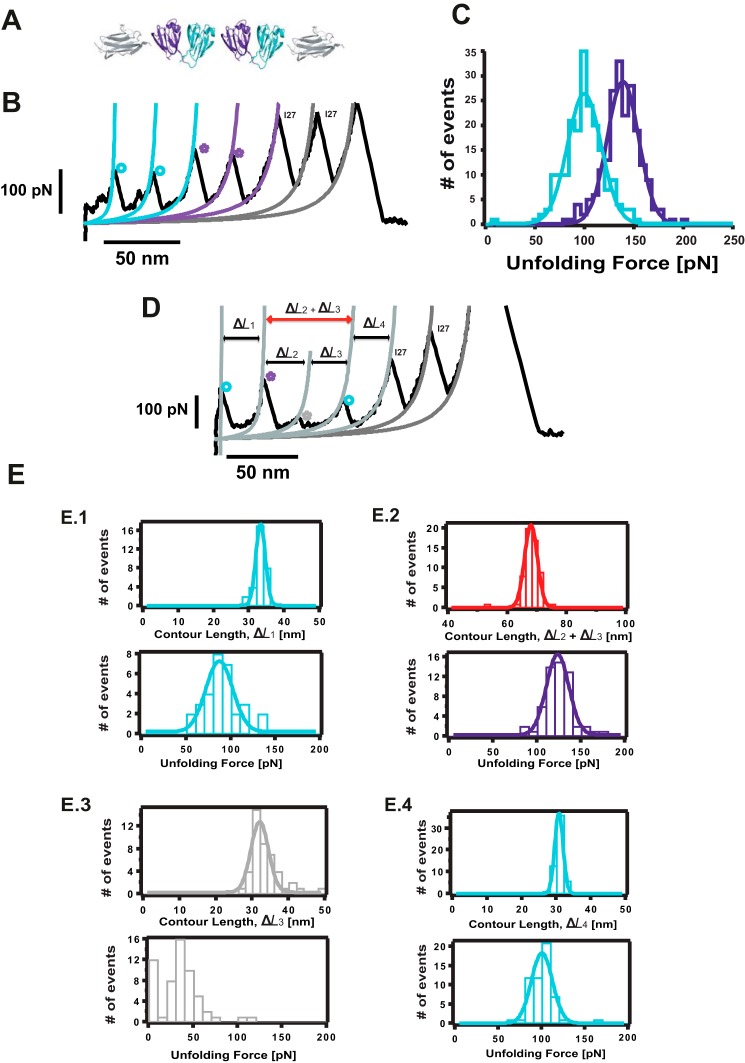
FIGURE 3.
Identification of a misfolded dimeric conformation in HγD-crys. A, schematic of the constructed [I27-(HγD-crys)2-I27] polyprotein. Mechanical unfolding of this construct results in two main types of trajectories. B, in ∼85% of instances, the Ctd (blue fit) unfolds first at 96.7 ± 17.0 pN (n = 198), followed by the unfolding of the Ntd (purple fit), at 135.8 ± 16.0 pN (n = 201) before the unfolding of the I27 marker occurring at 251.9 ± 34.0 pN (n = 158). D, in the remaining ∼15% of trajectories (n = 57), the [I27-(HγD-crys)2-I27] polyprotein unfolds in an anomalous fashion that is not consistent with a mechanical hierarchy scenario. E, a first unfolding peak (E.1) occurs at 82.2 ± 15.0 pN (n = 31) and ΔL1 = 32.3 ± 1.3 nm (n = 31). The second unfolding peak (purple star) occurs at 118.4 ± 12.8 pN and (ΔL2 + ΔL3) = 66.9 ± 2.1 nm; n = 57, E.2). Next, a low mechanical stability peak of ∼35 pN and ΔL3 = 31.0 ± 2.4 nm (n = 45) is observed. Prior to the unfolding of the I27 internal controls, a last unfolding event, characterized by an unfolding force of 95.3 ± 12.1 pN and a ΔL4 = 29.7 ± 1.2 nm (n = 56), is observed (E.4).