FIGURE 5.
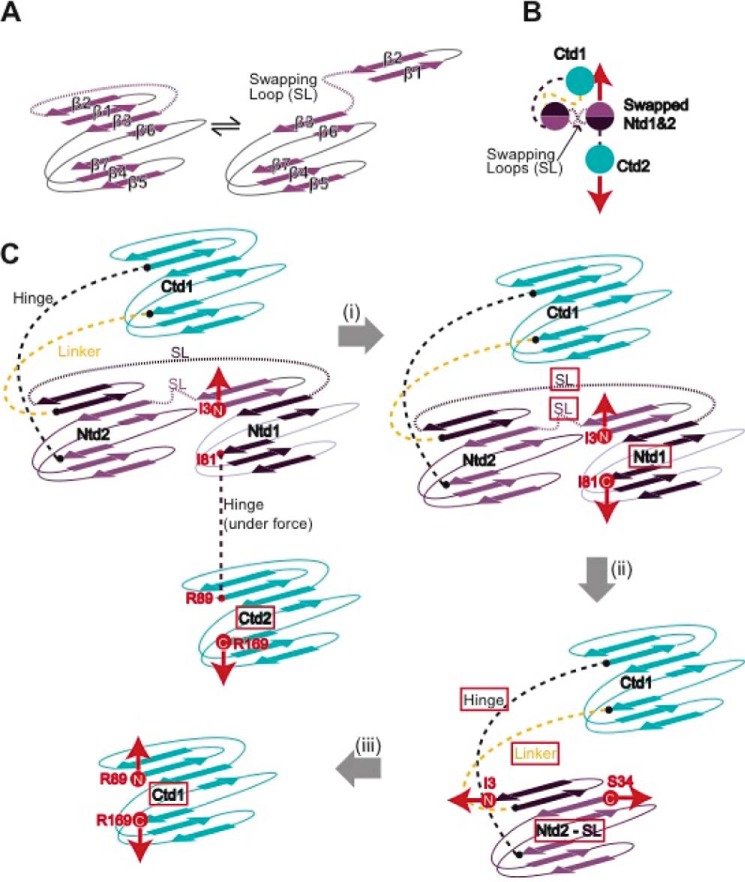
The most probable swapping motif involves the β1 and β2 strands of the Ntd of HγD-crys. A, topological model of the domain-swapped protein construct that identifies the swapping motifs between two Ntds (B) and highlights the sequence of structural rearrangements the polyprotein construct undergoes when exposed to mechanical force (C). After Ctd2 unfolding (i), the force is applied from the C-terminal of Ntd1 (I81) and the N terminus of the swapped conformation (I3). It is very possible that the swapping rearrangement accounts for the lower mechanostability (∼20 pN) of this swapped Ntd1 domain with respect to a WT Ntd (Fig. 2). When the Ntd1-swapped motif unfolds and extends concomitant to the swapping loop, the force is directly applied to the new N(I3) and C(S34) termini of the Ntd2 swapped conformation (ii). Remarkably, the pulling direction has now changed, which readily explains the lower mechanical stability of Ntd2 of the swapped structure. This plausible structural model is consistent with the experimental traces we observed (Fig. 3D) in terms of the unfolding sequence, increment in contour length, and measured unfolding force.