Abstract
Accumulating evidence has revealed that the expression of the lipid raft protein flotillin-1 is elevated in various human cancers, but the role flotillin-1 plays in the carcinogenesis of cervical cancer remains unclear. The expression profile of flotillin-1 was assayed using real-time PCR, western blotting, and immunohistochemical (IHC) staining in cervical cancer cell lines and cancer tissues with paired adjacent noncancerous cervical tissues. The expression of flotillin-1 protein was detected by IHC staining in a large cohort of 308 paraffin-embedded cervical cancer tissues. Ectopic expression and the short hairpin RNA interference approach were employed to determine the role of flotillin-1 in cervical cancer cell metastasis and the possible mechanism involved. Flotillin-1 expression protein and mRNA were significantly upregulated in cervical cancer cell lines and cancer tissues; elevated expression of flotillin-1 protein in early-stage cervical cancer was significantly associated with pelvic lymph node metastasis (P < 0.001), and was an independent predictive factor of poor overall survival. Moreover, flotillin-1 up- and downregulation remarkably affected cervical cancer cell motility and invasion, respectively, through epithelial-mesenchymal transition (EMT) regulated by the Wnt/β-catenin and nuclear factor-κB (NF-κB) pathways. Our results suggest that flotillin-1 facilitates cervical cancer cell metastasis through Wnt/β-catenin and NF-κB pathway-regulated EMT and that the flotillin-1 expression profile serves not only as novel predictor of pelvic lymph node metastasis, but also as neoteric risk factor for patients with early-stage cervical cancer.
Keywords: Flotillin-1, cervical cancer, lymph node metastasis, prognosis, EMT, Wnt/β-catenin and NF-κB pathways
Introduction
Cervical cancer is the fourth most common cancer and the fourth most frequent cause of cancer death in women globally, with 527,600 new cases and 265,700 deaths in 2012 [1]. Moreover, nearly 90% of new cases occur in developing countries [1]. In China, the largest developing country in the world and where neither nationwide screening programs nor vaccines against human papillomavirus are available, there were 87,982 new cases and 23,375 deaths from cervical cancer in 2011 [2], rendering cervical cancer one of the heaviest health burdens for Chinese women.
Although not included in the International Federation of Gynecology and Obstetrics (FIGO) staging system, it has been determined that retroperitoneal lymph node metastasis is the most important prognostic risk factor in early-stage cervical cancer (FIGO stage IB-IIA), which is an indication for adjuvant pelvic radiation plus concurrent cisplatin-based chemotherapy after primary surgery [3]. The survival rates of patients with stage I cancer are reported as 80-98%, but decrease dramatically to 50% when lymph node metastasis is present [4]. Unfortunately, sufficiently accurate models for predicting lymph node metastasis both preoperatively and intraoperatively remain beyond clinical usage, while the molecular mechanism of lymph node metastasis remains a conundrum.
Lipid rafts are stero- and sphingolipid-enriched small cell membrane microdomains that serve as signaling and sorting platforms for numerous molecules involved in various biological processes [5]. Flotillins, or reggies, have two homologous members: flotillin-1/reggie-2 and flotillin-2/reggie-1, and are scaffolding proteins of lipid rafts and are generally considered lipid microdomain markers [6]. Evolutionarily conserved and universally expressed, flotillins are involved in various cellular processes, including membrane protein recruiting, membrane receptor signaling, cell proliferation, cell adhesion, and as recently revealed, tumorigenesis [7-9]. In particular, flotillin-1 dysregulation has been found in several cancers of epithelial origin, namely breast cancer, hepatocellular carcinoma, and non-small cell lung cancer [10-13]. However, whether flotillin-1 dysregulation also occurs in cervical cancer and the possible role flotillin-1 plays in cervical cancer metastasis remains to be elucidated.
Material and methods
Patients and tissue specimens
The study cohort enrolled 308 patients with early-stage cervical cancer (FIGO stage IB-IIA) who underwent radical hysterectomy and lymphadenectomy at the Department of Gynecologic Oncology, the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital), Kunming, China, from January 2004 to December 2009. Patients who received neoadjuvant chemotherapy and/or radiation prior to surgery were excluded. Clinical stage was assessed according to the revised FIGO staging for carcinoma of the cervix [14]; tumor pathological type and differentiation grade were defined according to World Health Organization criteria [15]. Table 1 outlines the clinical/pathological features of the study cohort. The Human Ethics Committee and the Research Ethics Committee of the Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital) approved this study. All samples were obtained with the consent of each patient, and their privacy was maintained. Follow-up data were collected retrospectively through medical records.
Table 1.
Clinical/pathological characteristics of the study cohort (n = 308)
| Clinical/pathological Characteristics | No. of Patients (%) |
|---|---|
| Age | Years |
| Range | 20-71 |
| Mean | 41.7±9.1 |
| Median | 40 |
| SCCA, ng/ml* | |
| ≤ 1.5 | 191 (66.4) |
| > 1.5 | 97 (33.7) |
| FIGO stage | |
| IB1 | 173 (56.2) |
| IB2 | 74 (24.0) |
| IIA1 | 28 (9.1) |
| IIA2 | 33 (10.7) |
| Histological type | |
| Squamous cell carcinoma | 271 (88.0) |
| Adenocarcinoma | 21 (6.8) |
| Adenosquamous | 16 (5.2) |
| Differentiation grade | |
| G1 | 21 (6.9) |
| G2 | 118 (38.6) |
| G3 | 167 (54.6) |
| Tumor size, cm | |
| < 4 | 196 (65.3) |
| ≥ 4 | 104 (34.7) |
| Deep stromal Invasion | |
| No | 135 (45.9) |
| Yes | 159 (54.1) |
| Positive parametrium | |
| No | 301 (97.7) |
| Yes | 7 (2.3) |
| Positive surgical margin | |
| No | 286 (92.9) |
| Yes | 22 (7.1) |
| LVSI** | |
| No | 257 (83.4) |
| Yes | 51 (16.6) |
| Lymph node metastasis | |
| No | 257 (83.4) |
| Yes | 51 (16.6) |
| Follow up years | |
| Range | 0.1-11 |
| Mean | 6.0±2.1 |
| Median | 6 |
| Recurrence | |
| No | 266 (86.4) |
| Yes | 42 (13.6) |
| Vital status at follow-up | |
| Alive | 274 (88.6) |
| Death from CC | 34 (11.0) |
| Expression of flontilin-1 | |
| Low or none | 192 (60.8) |
| High | 116 (36.7) |
SCCA: Squamous cell carcinoma antigen.
LVSI: Lymphovascular space involvement.
Cell culture
Primary human foreskin keratinocyte (hKC) cells and four cervical cancer cell lines stored at our laboratory were originally obtained from American Type Culture Collection (Manassas, VA). HeLa, C33A, SiHa, and hKC cells were cultured in Eagle’s minimum essential medium (GIBCO BRL, Rockville, MD); Ca Ski cells were cultured in RPMI 1640 medium (GIBCO BRL). All cell lines were supplemented with 10% fetal bovine serum (FBS) (HyClone Laboratories, Logan, UT).
Vectors, retroviral infection, and transfection
Flotillin-1 overexpression and silencing plasmids were constructed as previously described [16], as was retroviral infection [16]. Stable cell lines expressing vector, flotillin-1, scramble, or flotillin-1 RNA interference (RNAi) were selected for 10 days with puromycin (0.5 μg/mL). Reporter plasmids containing wild-type (CCTTTGATC; TOP-flash) or mutant (CCTTTGGCC; FOP-flash) T cell factor/lymphocyte enhancer factor (TCF/LEF) DNA binding sites (Upstate Biotechnology, Lake Placid, NY) were used to quantitatively examine β-catenin/TCF activity. Phosphorylated nuclear factor-κB-luciferase (pNF-κB-luc) and control plasmids (Clontech, Mountain View, CA) were used to determine NF-κB activity. Transfection was performed using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocols.
RNA extraction and real-time quantitative PCR
Total RNA from cultured cells was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s manual. Complementary DNA (cDNA) was synthesized using M-MLV reverse transcriptase (Promega, Fitchburg, WI). Real-time PCR was performed using an ABI Prism 7500 Sequence Detection System (Applied Biosystems, Foster City, CA) using SYBR Green I dye (Roche, Penzberg, Germany). Expression data were normalized to the geometric mean of the housekeeping gene glyceraldehyde-3-phosphate (GAPDH) to control the variability in expression levels and were calculated as 2-[(Ct of gene)-(Ct of GAPDH)], where Ct represents the threshold cycle for each transcript. Table S1 lists the probe sequences used for the real-time PCR.
Western blotting
Western blotting was performed as previously described [16] with anti-flotillin-1 (Sigma-Aldrich, Saint Louis, MO) and anti-E-cadherin, anti-Vimentin and anti-N-cadherin (BD Transduction Laboratories, Lexington, UK) mouse monoclonal antibodies. The membranes were stripped and re-probed with anti-GAPDH mouse monoclonal antibody (Sigma-Aldrich) as the loading control.
Immunohistochemical (IHC) staining
IHC staining was performed as previously described [16] using Histostain-Plus Kits (Invitrogen) according to the manufacturer’s protocols. Known immunostaining-positive and -negative slides were used as the controls.
Evaluation of IHC staining
Two pathologists (W. Lin and C. Yang) blinded to the clinical covariates evaluated the flotillin-1 staining independently. The staining results were scored based on: (a) percentage of positive tumor cells in the tumor tissue: 0 (0%), 1 (1-25%), 2 (26-50%), 3 (51-75%), 4 (> 75%); and (b) staining intensity: 0 (no staining), 1 (weak staining), 2 (moderate staining), 3 (strong staining). The immunoreactivity score (IRS, ranging 0-12) was then calculated by multiplying the percentage score by the intensity score [17]; both nuclear and cytoplasmic expression were scored as positive staining. The final score was stratified as - (IRS = 0), + (IRS = 1-4), ++ (IRS = 5-8), and +++ (IRS = 9-12). In this study, - to + was considered low expression; ++ to +++ was considered high expression.
Transwell migration assay
Cells (1 × 104) were plated on the top side of Matrigel-coated polycarbonate Transwell filters (BD Biosciences, San Jose, CA) and incubated at 37°C for 20 h. Cells inside the upper chamber were removed with cotton swabs. Cells that migrated to the bottom surface of the membrane were fixed in 1% paraformaldehyde, stained with hematoxylin, and 10 random fields per well were counted under × 200 magnification. Three independent experiments were performed and the data are presented as the mean ± standard deviation (SD).
Wound healing assay
Cells were seeded on 6-well plates with Eagle’s minimum essential medium containing 10% FBS and grown to monolayer confluence. Straight wounds were created in the monolayers using a sterile pipette tip. Images were captured and documented at 0, 12, 24, and 36 h after wounding using an inverted (Olympus, Shinjuku, Tokyo, Japan) IX50 microscope with 10 × objective lens and Image-Pro Plus software (Media Cybernetics, Bethesda, MD). Each experiment was repeated three times.
Luciferase assay
Cells (3 × 104) were seeded in triplicate in 48-well plates and allowed to settle for 24 h. Reporter plasmid (0.1 μg) or control luciferase plasmid (0.1 μg) plus 1 ng pRL-TK Renilla plasmid (Promega) were transfected into the cells. At 48 h after transfection, luciferase and Renilla activity was measured using a Dual Luciferase Reporter Assay Kit (Promega).
Statistical analysis
The correlation between flotillin-1 expression and clinical/pathological characteristics was assessed using Pearson’s χ2 test and Spearman correlation analysis. Overall survival (OS) was defined as the time from diagnosis to the date of cancer-related death; progression-free survival (PFS) was defined as the time from diagnosis to the onset of recurrence. Survival curves were plotted using the Kaplan-Meier method and compared by the log-rank test. Multivariate analysis was performed for all parameters that were significant in the univariate analysis using the Cox regression model. All statistical analyses were carried out with SPSS software package (IBM, Armonk, NY, standard version 22.0). A 2-sided p-value < 0.05 was considered statistically significant.
Results
Flotillin-1 expression is significantly upregulated in cervical cancer cell lines and cervical cancer tissues
Both flotillin-1 protein and mRNA are significantly upregulated in the four cervical cancer cell lines (HeLa, Ca Ski, C33A, SiHa) and five fresh cervical cancer tissues as compared to the hKC cells and the paired adjacent noncancerous cervical tissues, respectively (Figure 1). IHC staining detected flotillin-1 protein in 93.8% (289/306) of paraffin-embedded cervical cancer tissues, while marginal or negative staining was detected in control normal cervical tissues and normal areas surrounding the cancerous tissues among all tumor sections (Figure 2A-H).
Figure 1.
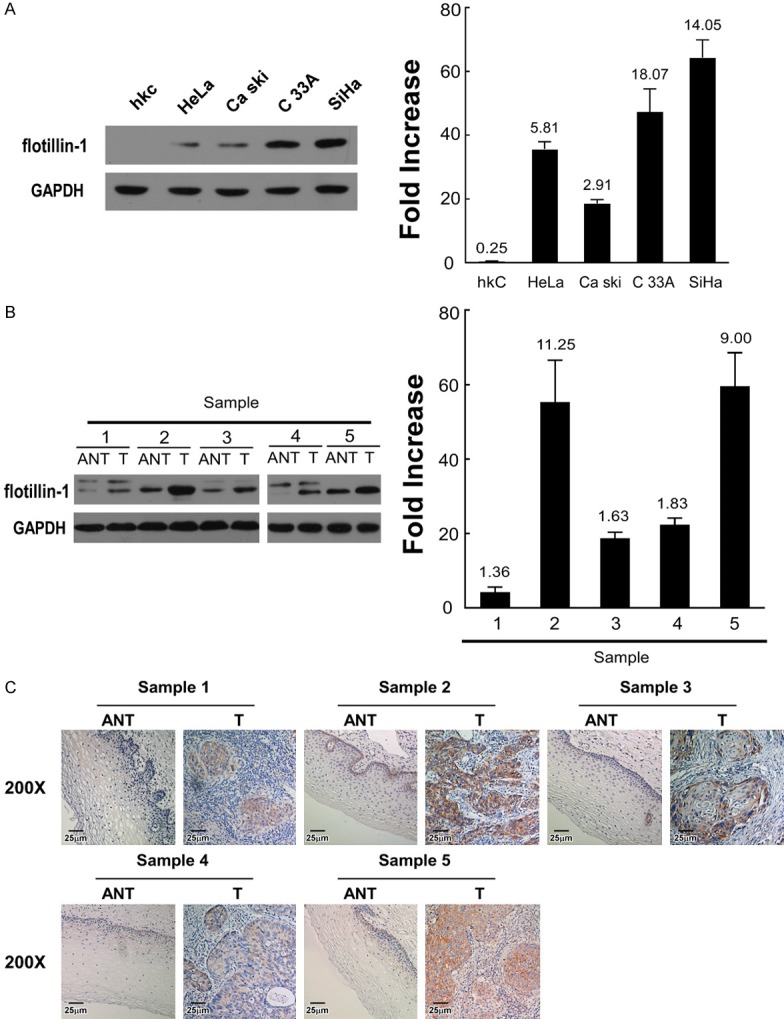
Expression of flotillin-1 protein and mRNA in cells and tissues. A. Expression of flotillin-1 protein (left) and mRNA (right) in hKC cells and HeLa, Ca Ski, C33A, and SiHa cervical cancer cells. B. Expression of flotillin-1 protein (left) and mRNA (right) in five paired cervical cancer (T) and adjacent noncancerous cervical tissues (ANT). Expression levels were normalized by GAPDH. Error bars represent SD calculated from three parallel experiments. C. IHC assay of flotillin-1 protein expression in five paired cervical cancer (T) and adjacent noncancerous cervical tissues (ANT).
Figure 2.
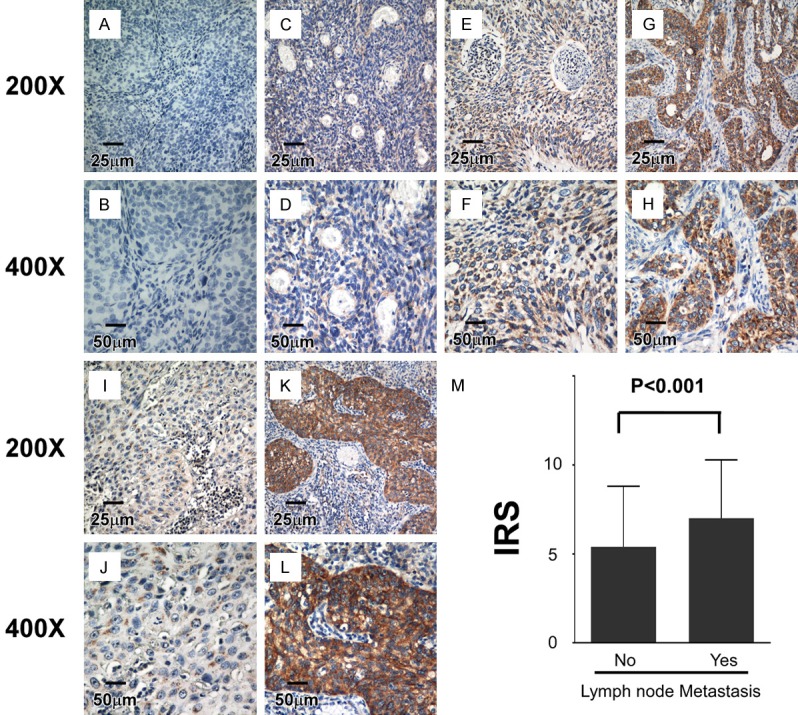
IHC assay of flotillin-1 protein expression in 308 paraffin-embedded cervical cancer tissues. A, B. Flotillin-1 expression was not detectable in cervical cancer tissues (this patient also did not present lymph node metastasis). C, D. Representative images of weak flotillin-1 staining in cervical cancer tissues. E, F. Representative images of moderate flotillin-1 staining in cervical cancer tissues. G, H. Representative images of strong flotillin-1 staining in cervical cancer tissues. I, J. Representative images of moderate flotillin-1 staining in cervical cancer tissues without lymph node metastasis. K, L. Representative images of strong flotillin-1 staining in cervical cancer tissues with lymph node metastasis. M. Average IRS of flotillin-1 in patients with and without lymph node metastasis. Error bars represent SD; p-value was calculated by Spearman correlation analysis.
Flotillin-1 protein expression profile correlates with pelvic lymph node metastasis in early-stage cervical cancer
We studied the correlation between the flotillin-1 protein expression profile and clinical/pathological characteristics in patients with early-stage cervical cancer further. There was no statistically significant correlation between flotillin-1 protein expression and age, squamous cell carcinoma antigen (SCCA) level, FIGO stage, differentiation grade, tumor size (cut-off, 4 cm), deep stromal invasion, positive parametrium, positive surgical margin, and lymphovascular space involvement (LVSI). However, flotillin-1 protein expression and histological type were significantly correlated (P = 0.031), and most importantly, lymph node metastasis (P < 0.001) was correlated (Table 2, Table S2; Figure 2I-M).
Table 2.
Clinical/pathological characteristics of patients with cervical cancer and flotillin-1 expression profile (n = 308)
| Characteristics | N (%) | Expression Level of flotillin-1 | χ2 test P | |
|---|---|---|---|---|
|
|
||||
| Low or non no. (%) | High no. (%) | |||
| Age (years) | 0.788 | |||
| ≤ 40 | 144 (46.8) | 86 (27.9) | 58 (18.8) | |
| > 40 | 164 (53.2) | 106 (34.4) | 58 (18.8) | |
| SCCA (ng/ml)* | 0.727 | |||
| ≤ 1.5 | 191 (66.4) | 124 (43.1) | 67 (23.3) | |
| > 1.5 | 97 (33.7) | 58 (20.1) | 39 (13.5) | |
| FIGO stage | 0.500 | |||
| IB1 | 173 (56.2) | 109 (35.4) | 64 (20.8) | |
| IB2 | 74 (24.0) | 49 (15.9) | 25 (8.1) | |
| IIA1 | 28 (9.1) | 14 (4.5) | 14 (4.5) | |
| IIA2 | 33 (10.7) | 20 (6.5) | 13 (4.2) | |
| Histological type | 308 | 0.082 | ||
| Squamous cell carcinoma | 271 (88.0) | 175 (56.8) | 96 (31.2) | |
| Adenocarcinoma | 21 (6.8) | 9 (2.9) | 12 (3.9) | |
| Adenosquamous | 16 (5.2) | 8 (2.6) | 8 (2.6) | |
| Differentiation grade | 0.636 | |||
| G1 | 21 (6.9) | 12 (3.9) | 9 (2.9) | |
| G2 | 118 (38.6) | 77 (25.2) | 41 (13.4) | |
| G3 | 167 (54.6) | 101 (33.0) | 66 (21.6) | |
| Tumor size, cm | 0.427 | |||
| < 4 | 196 (65.3) | 119 (39.7) | 77 (25.7) | |
| ≥ 4 | 104 (34.7) | 68 (22.7) | 36 (12.0) | |
| Deep stromal Invasion | 0.592 | |||
| No | 135 (45.9) | 87 (29.6) | 48 (1.3) | |
| Yes | 159 (54.1) | 96 (32.7) | 63 (21.4) | |
| Positive parametrium | 0.282 | |||
| No | 301 (97.7) | 189 (61.4) | 112 (36.4) | |
| Yes | 7 (2.3) | 3 (1.0) | 4 (1.3) | |
| Positive surgical margin | 0.896 | |||
| No | 286 (92.9) | 178 (58.8) | 108 (35.1) | |
| Yes | 22 (7.1) | 14 (4.5) | 8 (2.6) | |
| LVSI** | 0.681 | |||
| No | 257 (83.4) | 184 (58.4) | 110 (25.0) | |
| Yes | 51 (16.6) | 8 (3.9) | 6 (12.7) | |
| Lymph node metastasis | < 0.001 | |||
| No | 257 (83.4) | 180 (58.4) | 77 (25.0) | |
| Yes | 51 (16.6) | 12 (3.9) | 39 (12.7) | |
| Recurrence | 0.685 | |||
| No | 266 (86.4) | 167 (70.2) | 99 (29.8) | |
| Yes | 42 (13.6) | 25 (43.9) | 17 (56.1) | |
| Vital status | < 0.001 | |||
| Alive | 274 (88.6) | 183 (59.4) | 91 (29.5) | |
| Death from CC | 34 (11.0) | 9 (2.9) | 25 (8.1) | |
SCCA: Squamous cell carcinoma antigen.
LVSI: Lymph vascular space involvement.
Elevated expression of flotillin-1 protein predicts poor OS in patients with early-stage cervical cancer
In this cohort, the OS rate of patients with high flotillin-1 expression was significantly lower than that of patients with low flotillin-1 expression (P < 0.001, log-rank test, Figure 3A). The same pattern was detected in patients with squamous cell carcinoma of the cervix (P < 0.001, log-rank test, Figure 3B). Even in patients without lymph node metastasis (P = 0.012, log-rank test, Figure 3C) or without recurrence (P < 0.01, log-rank test, Figure 3D), low flotillin-1 expression also demonstrated a survival advantage over high flotillin-1 expression. However, no impact of flotillin-1 expression on PFS was detected in this study cohort (Figure S1). The multivariate Cox regression model revealed that pelvic lymph node metastasis (relative risk: 3.304, confidence interval [CI]: 1.581-6.901, P = 0.021), site of recurrence (relative risk: 3.179, CI: 2.345-4.308, P = 0.045), and flotillin-1 expression level (relative risk: 3.423, CI: 1.495-7.837, P = 0.025) were independent prognostic factors for poor OS (Table 3).
Figure 3.
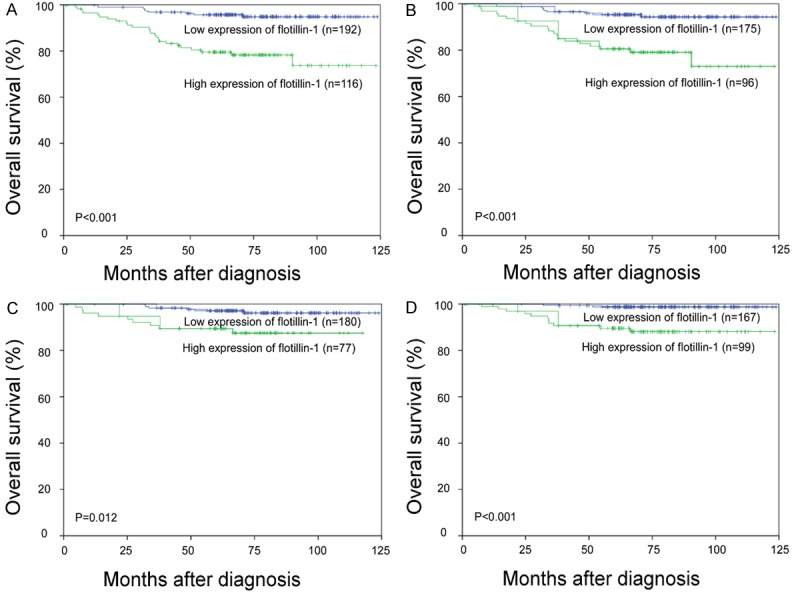
Kaplan-Meier curves with univariate analyses (log-rank) in cervical cancer patients with low versus high flotillin-1 expression. A. OS curves of all patients. B. OS curves of patients with squamous cell carcinoma; C. OS curves of patients without lymph node metastasis. D. OS curves of patients without recurrence.
Table 3.
Cox regression model univariate and multivariate analyses of prognostic parameters in patients with cervical cancer
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| No. patients | p | Regression coefficient (SE) | p | Relative risk | 95% confidence interval | |
| Lymph node metastasis | 0.021 | 1.180 (0.512) | 0.001 | 3.304 | 1.581-6.901 | |
| No | 254 | |||||
| Yes | 51 | |||||
| Site of recurrence | 0.045 | 0.852 (0.425) | < 0.001 | 3.179 | 2.345-4.308 | |
| No | 266 | |||||
| Local | 13 | |||||
| Distant | 19 | |||||
| Both | 10 | |||||
| Expression Level of flotillin-1 | 0.025 | 1.117 (0.499) | 0.004 | 3.423 | 1.495-7.837 | |
| Low or none | 192 | |||||
| High | 116 | |||||
Flotillin-1 promotes cervical cancer cell motility and invasion through Wnt/β-catenin and NF-κB pathway-regulated epithelial-mesenchymal transition (EMT)
To further identify the role of flotillin-1 in cervical cancer metastasis and the possible mechanism, flotillin-1 cDNA and two flotillin-1 short hairpin RNAs (shRNAs) were used to respectively express flotillin-1 ectopically and to suppress endogenous flotillin-1 stably in HeLa and SiHa cells. As expected, the Transwell migration assay and wound healing assay both indicated that, compared to cells transfected with control vectors, ectopic expression of flotillin-1 strikingly enhanced HeLa and SiHa cell motility and invasion, while flotillin-1 ablation dramatically inhibited it (Figure 4A and 4B). Western blotting further revealed that flotillin-1 overexpression enhanced the expression of mesenchymal markers including Vimentin and N-cadherin, but inhibited the expression of epithelial markers, such as E-catenin (Figure 4C), while knockdown of flotillin-1 reversed it (Figure 4C). The TOP-flash/FOP-flash Wnt reporter assay confirmed that the canonical (β-catenin-dependent) Wnt pathway was highly activated in cells expressing ectopic flotillin-1 and was inactivated in cells with suppressed endogenous flotillin-1 expression (Figure 5A). In addition, the expression levels of a series of Wnt/β-catenin target genes were upregulated following flotillin-1 overexpression and were downregulated when flotillin-1 was silenced (Figure 5B). Moreover, ectopic expression of flotillin-1 significantly enhanced the NF-κB luciferase activity as well as the expression levels of a number of well-known downstream genes of the NF-κB pathway, while the ablation of flotillin-1 reduced it (Figure 5C and 5D).
Figure 4.
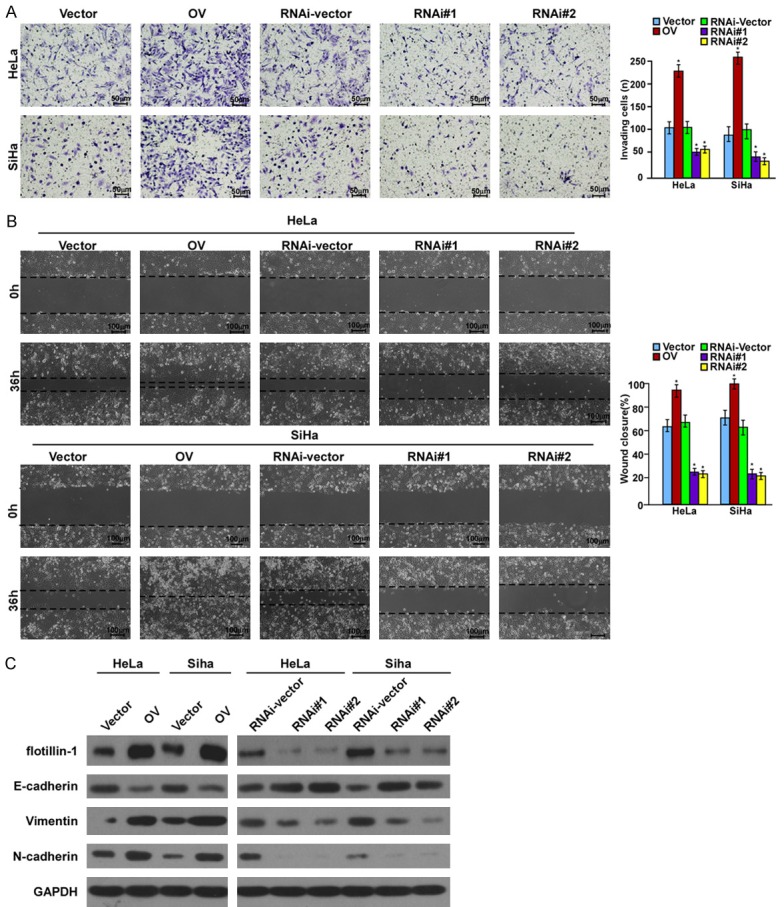
Flotillin-1 promotes cervical cancer cell motility and invasion through EMT. A. Invasion ability induced by FBS as analyzed by Transwell migration assay (× 200, *P < 0.001). B. Motility as measured by testing the wound closure rate at 0 and 36 h (× 200, *P < 0.001)). C. Western blotting analysis of the expression of flotillin-1 and EMT markers. Vector: Cells transfected with vector; OV: Cells transfected with flotillin-1 cDNA; RNAi-vector: cells transfected with scramble shRNA; RNAi#1/RNAi#2: cells transfected with two human shRNA sequences to repress flotillin-1.
Figure 5.
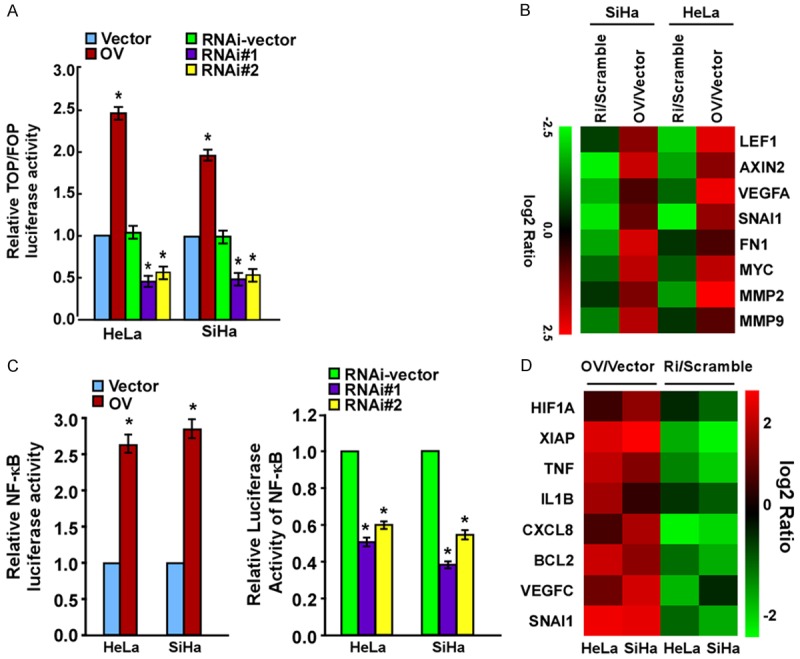
Flotillin-1 activates the NF-κB and Wnt/β-catenin pathways in cervical cancer cells. A. Luciferase reporter activities analyzed in cells according to the TOP-flash versus FOP-flash intensity scale. B. Real-time PCR indicates an apparent overlap between Wnt/β-catenin-dependent gene expression and flotillin-1-regulated gene expression. Pseudo-color represents the intensity scale of flotillin-1 shRNA versus RNAi vector or flotillin-1 cDNA vs. Vector, generated by log2 transformation. C. NF-κB luciferase reporter activity. D. Real-time PCR indicates an apparent overlap between NF-κB-dependent gene expression and flotillin-1-regulated gene expression. Pseudo-color represents the intensity scale of flotillin-1 cDNA vs. Vector or flotillin-1 shRNA versus RNAi-vector, generated by log2 transformation. Vector: cells transfected with vector; OV: cells transfected with flotillin-1 cDNA; RNAi-vector/Scramble: cells transfected with scramble shRNA; RNAi#1/RNAi#2/Ri: cells transfected with two human shRNA sequences to repress flotillin-1.
Discussion
This is the first report to demonstrate that flotillin-1 expression is significantly upregulated in cervical cancer cell lines and cancer tissues at both protein and mRNA level in comparison with that of hKC cells and paired adjacent noncancerous cervical tissues. Elevated expression of flotillin-1 protein is associated with pelvic lymph node metastasis, and the expression profile of flotillin-1 serves as an independent prognostic factor that predicts poor OS time in patients with early-stage cervical cancer even when there is no lymph node metastasis or recurrence. Moreover, the up- and downregulation of flotillin-1 remarkably affected cervical cancer cell motility and invasion, respectively, possibly through Wnt/β-catenin and NF-κB pathway-regulated EMT. These data imply that flotillin-1 may facilitate cervical cancer metastasis and that the flotillin-1 expression profile acts not only as a novel predictor of pelvic lymph node metastasis, but also as neoteric prognostic risk factor for patients with early-stage cervical cancer.
Radical hysterectomy plus bilateral pelvic lymph node dissection with (or without) para-aortic lymph node sampling remains the standard primary treatment for patients with early-stage cervical cancer (FIGO stage IB-IIA), as retroperitoneal lymph node metastasis is the major prognostic risk factor that determines whether a patient needs adjuvant pelvic radiation plus concurrent cisplatin-based chemotherapy after surgery [3,18]. In fact, the occurrence of postoperative complications caused by lymphadenectomy, especially pelvic lymphocyte, can reach 25%, while oncologists increasingly value the potential anti-cancer immunity of intact lymph nodes [4]. Unfortunately, there are no accurate and efficient models for gynecologic oncologists to evaluate pelvic lymph node metastasis both preoperatively and intraoperatively. Although recent data suggest that sentinel lymph node mapping and biopsy may be the most promising method [19,20], whether the technique has been sufficiently validated for routine use is still debated [21,22]. In the present study, our data demonstrate that the expression level of flotillin-1 protein is significantly associated with lymph node metastasis in early-stage cervical cancer, shedding light on the possibility of predicting retroperitoneal lymph node metastasis preoperatively by investigating flotillin-1 protein expression levels in biopsy samples, subsequently allowing the adoption of a more personalized treatment algorithm. For patients with significantly elevated flotillin-1 protein expression, implying high lymph node metastasis risk, definitive radiation with concurrent cisplatin-containing chemotherapy instead of surgery should be advised to prevent overlying complications; for those with low flotillin-1 protein expression, suggesting low lymph node metastasis risk, radical hysterectomy alone could be considered to preserve intact lymph nodes, and further, anti-cancer immunity.
Furthermore, the univariate and multivariate analyses suggested that the level of flotillin-1 protein expression is an independent prognostic factor that predicts poor OS in early-stage cervical cancer patients, even in patients without lymph node metastasis or recurrence, suggesting that patients with high flotillin-1 protein expression may require more intensive treatment to obtain a desirable prognosis and closer surveillance to detect early recurrence even when there are no other risk factors, such as lymph node metastasis, at the time.
Flotillin-1 encodes a caveolae-associated integral membrane protein that belongs to the lipid raft family, and is involved in vesicular trafficking and signal transduction [23]. Quite recently, an increasing number of publications have reported the essential roles of flotillin-1 in tumorigenesis. Flotillin-1 overexpression predicts poor prognosis in hepatocellular carcinoma [12,24], non-small cell lung cancer [25], soft tissue sarcomas [25], tongue squamous cell cancer [26], clear cell renal cell carcinoma [27], and gastric cancer [28]; silencing flotillin-1 inhibits breast cancer cell proliferation and tumorigenicity both in vitro and in vivo [11], while flotillin-1 depletion leads to ErbB2 and ErbB3 internalization and degradation [29,30]. Moreover, flotillin-1 promotes cell growth and metastasis by activating the NF-κB signaling pathway in both esophageal squamous cell carcinoma cells and oral squamous cell carcinoma cells [16,31]. In the present study, there was elevated flotillin-1 expression in cervical cancer cell lines and cancer tissues compared to hKC cells and normal cervical tissues, whereas cervical cancer cell motility and invasion were strikingly enhanced following the ectopic expression of flotillin-1, and were dramatically inhibited by the ablation of flotillin-1 as compared to cells transfected with control vectors, suggesting that flotillin-1 may play an oncogenic role in cervical cancer as well.
EMT contributes to metastasis of a variety of human cancer cells [32]. Multiple signaling pathways regulate EMT, with the NF-κB and Wnt/β-catenin pathways playing a pivotal role [32]. In agreement with previous reports [16,31], we found that flotillin-1 facilitated cancer cell metastasis through NF-κB pathway-regulated EMT; activation of the canonical (β-catenin-dependent) Wnt pathway, which is active in cervical cancer [33], may also play a role in flotillin-1-induced EMT. However, the detailed molecular mechanism underlying flotillin-1 interaction with the Wnt/β-catenin and NF-κB pathways to induce EMT in cervical cancer cells, which facilitates their metastasis, requires further investigation in our subsequent studies.
Taken together, our data first demonstrate that flotillin-1 expression is significantly upregulated in early-stage cervical cancer and that elevated flotillin-1 is significantly associated with pelvic lymph node metastasis and poor OS. Flotillin-1 promoted cervical cancer cell motility and invasion via Wnt/β-catenin and NF-κB pathway-regulated EMT. Our data first suggest that flotillin-1 may serve not only as a novel predictor of pelvic lymph node metastasis, but also as a neoteric prognostic risk factor for patients with early-stage cervical cancer.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81402146), the Research Fund for the Doctoral Program of Higher Education of China (20125317120002), China Scholarship Council (201408535054), Science and Technology Planning Project of Yunnan Province (2014FB195), and the Scientific Research Foundation for Doctors of The Third Affiliated Hospital of Kunming Medical University (Yunnan Tumor Hospital) (BSJJ201402).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Zeng H, Zhang S, He J. Annual report on status of cancer in China, 2011. Chin J Cancer Res. 2015;27:2–12. doi: 10.3978/j.issn.1000-9604.2015.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barakat R, Berchuck A, Markman M, Randall ME. Principles and Practice of Gynecologic Oncology. Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 4.Benedetti Panici P, Basile S, Angioli R. Pelvic and aortic lymphadenectomy in cervical cancer: the standardization of surgical procedure and its clinical impact. Gynecol Oncol. 2009;113:284–290. doi: 10.1016/j.ygyno.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Pike LJ. Rafts defined: a report on the Keystone Symposium on Lipid Rafts and Cell Function. J Lipid Res. 2006;47:1597–1598. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Schulte T, Paschke KA, Laessing U, Lottspeich F, Stuermer CA. Reggie-1 and reggie-2, two cell surface proteins expressed by retinal ganglion cells during axon regeneration. Development. 1997;124:577–587. doi: 10.1242/dev.124.2.577. [DOI] [PubMed] [Google Scholar]
- 7.Zhao F, Zhang J, Liu YS, Li L, He YL. Research advances on flotillins. Virol J. 2011;8:479. doi: 10.1186/1743-422X-8-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banning A, Tomasovic A, Tikkanen R. Functional aspects of membrane association of reggie/flotillin proteins. Curr Protein Pept Sci. 2011;12:725–735. doi: 10.2174/138920311798841708. [DOI] [PubMed] [Google Scholar]
- 9.Staubach S, Hanisch FG. Lipid rafts: signaling and sorting platforms of cells and their roles in cancer. Expert Rev Proteomics. 2011;8:263–277. doi: 10.1586/epr.11.2. [DOI] [PubMed] [Google Scholar]
- 10.Donatello S, Babina IS, Hazelwood LD, Hill AD, Nabi IR, Hopkins AM. Lipid raft association restricts CD44-ezrin interaction and promotion of breast cancer cell migration. Am J Pathol. 2012;181:2172–2187. doi: 10.1016/j.ajpath.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin C, Wu Z, Lin X, Yu C, Shi T, Zeng Y, Wang X, Li J, Song L. Knockdown of FLOT1 impairs cell proliferation and tumorigenicity in breast cancer through upregulation of FOXO3a. Clin Cancer Res. 2011;17:3089–3099. doi: 10.1158/1078-0432.CCR-10-3068. [DOI] [PubMed] [Google Scholar]
- 12.Zhang SH, Wang CJ, Shi L, Li XH, Zhou J, Song LB, Liao WT. High Expression of FLOT1 Is Associated with Progression and Poor Prognosis in Hepatocellular Carcinoma. PLoS One. 2013;8:e64709. doi: 10.1371/journal.pone.0064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arkhipova KA, Sheyderman AN, Laktionov KK, Mochalnikova VV, Zborovskaya IB. Simultaneous expression of flotillin-1, flotillin-2, stomatin and caveolin-1 in non-small cell lung cancer and soft tissue sarcomas. BMC Cancer. 2014;14:100. doi: 10.1186/1471-2407-14-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banning A, Regenbrecht CR, Tikkanen R. Increased activity of mitogen activated protein kinase pathway in flotillin-2 knockout mouse model. Cell Signal. 2014;26:198–207. doi: 10.1016/j.cellsig.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumours of the Female Reproductive Organs (IARC WHO Classification of Tumours) 4th Edition. World Health Organization; 2014. [Google Scholar]
- 16.Song L, Gong H, Lin C, Wang C, Liu L, Wu J, Li M, Li J. Flotillin-1 promotes tumor necrosis factor-alpha receptor signaling and activation of NF-kappaB in esophageal squamous cell carcinoma cells. Gastroenterology. 2012;143:995–1005. e1012. doi: 10.1053/j.gastro.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Remmele W, Stegner HE. [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue] . Pathologe. 1987;8:138–140. [PubMed] [Google Scholar]
- 18.Cervical Cancer. Version 2 2015. NCCN.org. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) [Google Scholar]
- 19.Cormier B, Diaz JP, Shih K, Sampson RM, Sonoda Y, Park KJ, Alektiar K, Chi DS, Barakat RR, Abu-Rustum NR. Establishing a sentinel lymph node mapping algorithm for the treatment of early cervical cancer. Gynecol Oncol. 2011;122:275–280. doi: 10.1016/j.ygyno.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecuru F, Mathevet P, Querleu D, Leblanc E, Morice P, Darai E, Marret H, Magaud L, Gillaizeau F, Chatellier G, Dargent D. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: results of the SENTICOL study. J. Clin. Oncol. 2011;29:1686–1691. doi: 10.1200/JCO.2010.32.0432. [DOI] [PubMed] [Google Scholar]
- 21.Altgassen C, Hertel H, Brandstadt A, Kohler C, Durst M, Schneider A. Multicenter validation study of the sentinel lymph node concept in cervical cancer: AGO Study Group. J. Clin. Oncol. 2008;26:2943–2951. doi: 10.1200/JCO.2007.13.8933. [DOI] [PubMed] [Google Scholar]
- 22.Bats AS, Buenerd A, Querleu D, Leblanc E, Darai E, Morice P, Marret H, Gillaizeau F, Mathevet P, Lecuru F. Diagnostic value of intraoperative examination of sentinel lymph node in early cervical cancer: a prospective, multicenter study. Gynecol Oncol. 2011;123:230–235. doi: 10.1016/j.ygyno.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. doi: 10.1074/jbc.272.21.13793. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Lv JY, Shi JF, Yang M, Liu SH, Li ZW, Wang HB, Zhang SG, Liu ZW, Ding JB, Xu DP, Zhao JM. Targeting the raft-associated Akt signaling in hepatocellular carcinoma. Biomed Res Int. 2014;2014:836025. doi: 10.1155/2014/836025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang C, Cicek MS, Charbonneau B, Kalli KR, Armasu SM, Larson MC, Konecny GE, Winterhoff B, Fan JB, Bibikova M, Chien J, Shridhar V, Block MS, Hartmann LC, Visscher DW, Cunningham JM, Knutson KL, Fridley BL, Goode EL. Tumor hypomethylation at 6p21.3 associates with longer time to recurrence of high-grade serous epithelial ovarian cancer. Cancer Res. 2014;74:3084–3091. doi: 10.1158/0008-5472.CAN-13-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Zhang Y, Chen SW, Li FJ, Zhuang SM, Wang LP, Zhang J, Song M. Prognostic significance of Flotillin1 expression in clinically N0 tongue squamous cell cancer. Int J Clin Exp Pathol. 2014;7:996–1003. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Li J, Song Y, Chen F, Pei Y, Yao F. Flotillin-1 expression in human clear-cell renal cell carcinoma is associated with cancer progression and poor patient survival. Mol Med Rep. 2014;10:860–866. doi: 10.3892/mmr.2014.2310. [DOI] [PubMed] [Google Scholar]
- 28.Gao W, Xu J, Wang F, Zhang L, Peng R, Shu Y, Wu J, Tang Q, Zhu Y. Plasma membrane proteomic analysis of human Gastric Cancer tissues: revealing flotillin 1 as a marker for Gastric Cancer. BMC Cancer. 2015;15:367. doi: 10.1186/s12885-015-1343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pust S, Klokk TI, Musa N, Jenstad M, Risberg B, Erikstein B, Tcatchoff L, Liestol K, Danielsen HE, van Deurs B, Sandvig K. Flotillins as regulators of ErbB2 levels in breast cancer. Oncogene. 2013;32:3443–3451. doi: 10.1038/onc.2012.357. [DOI] [PubMed] [Google Scholar]
- 30.Asp N, Pust S, Sandvig K. Flotillin depletion affects ErbB protein levels in different human breast cancer cells. Biochim Biophys Acta. 2014;1843:1987–1996. doi: 10.1016/j.bbamcr.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Xiong P, Xiao LY, Yang R, Guo Q, Zhao YQ, Li W, Sun Y. Flotillin-1 promotes cell growth and metastasis in oral squamous cell carcinoma. Neoplasma. 2013;60:395–405. doi: 10.4149/neo_2013_051. [DOI] [PubMed] [Google Scholar]
- 32.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 33.Ford CE, Henry C, Llamosas E, Djordjevic A, Hacker N. Wnt signalling in gynaecological cancers: A future target for personalised medicine? Gynecol Oncol. 2016;140:345–51. doi: 10.1016/j.ygyno.2015.09.085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


