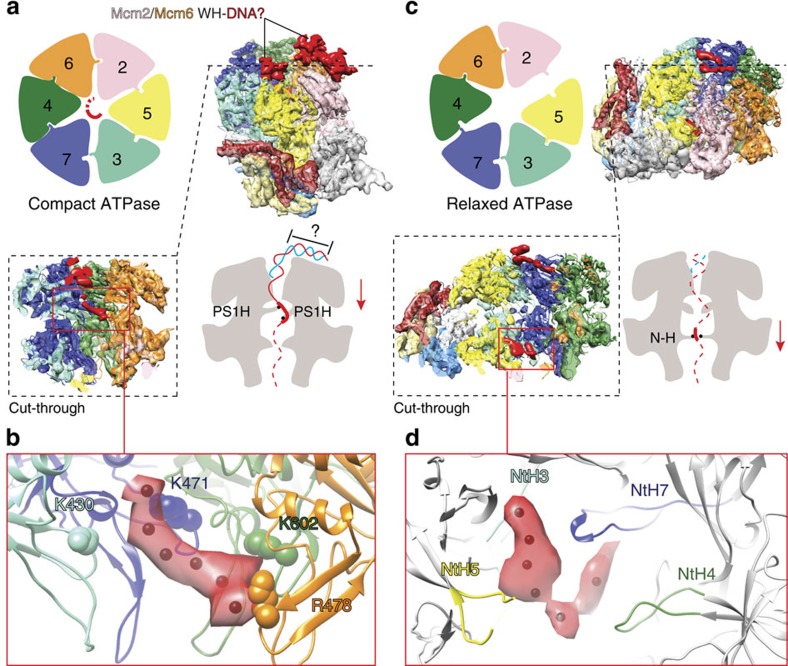
Figure 4. DNA-bound form of the CMG helicase.
(a) The compact ATPase form contains rod-shaped, bent density features surmounting the ATPase face tentatively assigned to duplex DNA engaged by the MCM winged helix (WH) C-terminal extensions. An MCM slice through the side view reveals an extended density feature, which we assign to single-stranded DNA, traversing the AAA+ pore. (b) Single-stranded DNA contacts conserved positively charged residues on the AAA+ PS1 hairpins that have a key role in DNA unwinding. (c) The relaxed ATPase form contains a thin density feature surmounting the AAA+ ring, which we assign to the flexible C-terminal MCM WH extensions. Although the AAA+ tier appears substrate free, a well-resolved elongated density threads through the MCM N-terminal collar and (d) contacts a set of N-terminal hairpins important for DNA binding and helicase activity.