Figure 6.
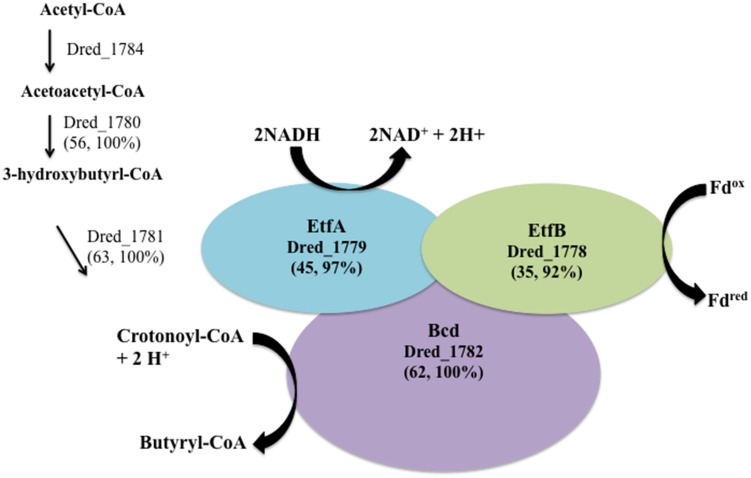
The cluster of proteins most increased on Fe(III)-citrate relative to pyruvate fermentation has similarity to a described FBEB system. A significant number of peptides and overall increased abundance was observed for proteins within hdr-containing locus VII (Dred_1778-84) on the Fe(III)-citrate condition. Genes within this locus are orthologs to the FBEB complex BcdA–EtfBC from Clostridium kluyveri DSM 555 (Li et al., 2008; Buckel and Thauer, 2013). Similarity between this complex (CKL_0454-8) and proteins in D. reducens is displayed as percent sequence identity across percent query coverage (http://blast.ncbi.nlm.nih.gov). Furthermore, redundant genes for each of the proteins involved in the FBEB step are expressed solely on the Fe(III)-citrate condition and include the electron transfer flavoproteins Dred_0573 and Dred_0572 (45, 97% and 31, 80%), and the acyl-CoA dehydrogenase domain-containing protein Dred_0402 (53, 100%). Furthermore, the enoyl-CoA hydratase/isomerase Dred_0401 is a redundant protein (60, 100%) for the step leading to crotonoyl-CoA, also unique to the Fe(III)-citrate condition.