Abstract
Effective disease management can benefit from mathematical models that identify drivers of epidemiological change and guide decision-making. This is well illustrated in the host–parasite system of sea lice and salmon, which has been modelled extensively due to the economic costs associated with sea louse infections on salmon farms and the conservation concerns associated with sea louse infections on wild salmon. Consequently, a rich modelling literature devoted to sea louse and salmon epidemiology has been developed. We provide a synthesis of the mathematical and statistical models that have been used to study the epidemiology of sea lice and salmon. These studies span both conceptual and tactical models to quantify the effects of infections on host populations and communities, describe and predict patterns of transmission and dispersal, and guide evidence-based management of wild and farmed salmon. As aquaculture production continues to increase, advances made in modelling sea louse and salmon epidemiology should inform the sustainable management of marine resources.
Keywords: Atlantic salmon, ecological modelling, emerging infectious disease, fish farm, marine disease, Pacific salmon
1. Introduction
Reductions in marine fish abundance due to overfishing are predicted to reduce pathogen diversity by ‘fishing-out parasites’ [1,2]. At the same time, the expansion of industrial aquaculture over the past 50 years has created new pathways and reservoirs for pathogens [3,4]. Farmed fish are often exposed to pathogens from sympatric wild fish populations, but are sheltered from many of the natural processes (e.g. predation [5], competition [6], migration [7,8]) that can remove infected individuals from populations. Farmed host populations are typically held at higher densities than populations in the wild, increasing opportunities for infections to spread. Understanding the effects of anthropogenic changes on marine disease in wild and farmed host populations is therefore a conservation and management priority.
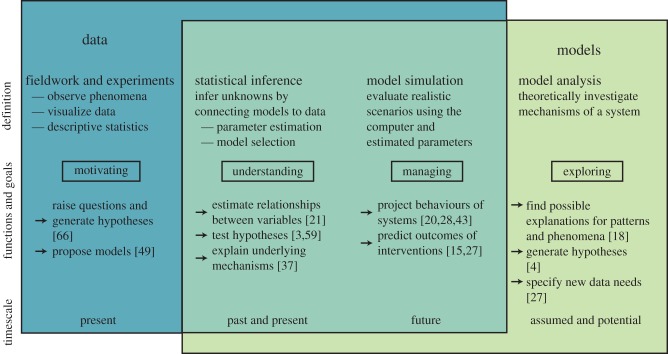
Mathematical models can inform marine conservation and management by addressing questions such as: When can a pathogen invade a population? What impact will it have on the population? Will it cause a chronic or acute infection? and How do ecological, anthropogenic and environmental drivers influence these patterns? While many questions in marine epidemiology remain unsolved, a growing body of quantitative research has tackled the interface of anthropogenic change, pathogen transmission and effects of infection on host populations. These models can test hypotheses, explain patterns or project management outcomes (figure 1) and can span the individual, population, community or ecosystem.
Figure 1.
Data and models are key components in the scientific process. Although data and models can be considered independently, the greatest advances in understanding the ecology and epidemiology of salmon and sea lice have been made by confronting models with data through statistical inference and model simulation. (Online version in colour.)
Progress in the field of marine epidemiology is well demonstrated by studies of sea louse infections. Sea lice (Lepeophtheirus salmonis and Caligus spp.) infecting salmon (Oncorhynchus spp. and Salmo spp.) have gained considerable attention (reviewed here and in [9–11]) due to economic costs associated with sea louse infections on salmon farms [12] and conservation concerns associated with sea louse infections on wild salmon [13]. Many models have been developed to study this system, ranging from statistical to dynamic and theoretical to applied. These models borrow insight from ecology, oceanography, epidemiology and physics (table 1). The complexity of salmon and sea louse epidemiology is such that no single mathematical model can capture it. Model types range from nonlinear dynamical systems, to regression, to individual-based simulation, to numerical ocean-circulation calculations, reflecting the broad spectrum of approaches to investigation. In short, sea lice and salmon make a convenient system for cataloguing the strategies used to model marine diseases.
Table 1.
Types of models and their application to the salmon sea louse pathosystem. Key references are included.
| model form | characteristics | applications and examples |
|---|---|---|
| difference equations | —discrete-time dynamics —spatially homogeneous —deterministic —can include delays |
population growth models, e.g. Ricker stock–recruitment relationship [14]; often used within other models —effects of lea louse infection and predation on salmon productivity [15] |
| ordinary differential equations | —continuous-time dynamics —spatially homogeneous —deterministic —can include delays |
host–parasite models for louse-salmon dynamics, e.g. Anderson–May model [16] and extensions thereof to incorporate predation —effects of treatments on sea louse populations on farms [17] —parasite-mediated changes to predation [18] |
| partial differential equations | —continuous-time dynamics —spatially heterogeneous —deterministic |
advection-diffusion models —sea louse dispersal from salmon farms along wild salmon migration routes [19] |
| matrix models (Leslie matrix, population projection matrix) | —linear system of difference equations —can include stochastic effects |
age-structured population growth models —analysis of temperature-dependent sea louse demography [20] |
| regression models | —statistical —descriptive/correlational —can include spatial effects |
GLM, GLMM, random effects, logistic regression —identifying epidemiological factors effecting sea lice abundance on salmon farms [21] —associations between aquaculture and sea louse infections on sea trout [22] |
| survival functions and hazard functions | —statistical —descriptive/correlational |
survival analysis —impacts of sea lice on salmon survival in the NE Atlantic [3] —effects of salinity on sea louse survival on juvenile salmon [23] |
| stochastic processes | —discrete-time or continuous-time dynamics —stochastic —can include additional hierarchy |
stochastic population growth models, e.g. stochastic Ricker model —hierarchical models of Pacific salmon productivity in relation to sea lice [24–26] |
| individual-based (or agent-based) model | —computer model —simulates actions and interactions of individuals within a system —can be deterministic or stochastic |
predicting benefits of cleaner fish in control of sea louse populations [27] |
| numerical ocean-circulation model | —numerically solved complex dynamical system; includes hydrodynamic equations, three-dimensional transport and diffusion equations) —realistic model for oceanic motion |
model for current, temperature and salinity patterns in marine environment —finite volume coastal ocean model (FVCOM) simulation of the spread of sea lice from salmon farms in British Columbia, Canada [28] —SINMOD simulation of sea louse and salmonid pancreatic disease virus in Norwegian fjords [29] |
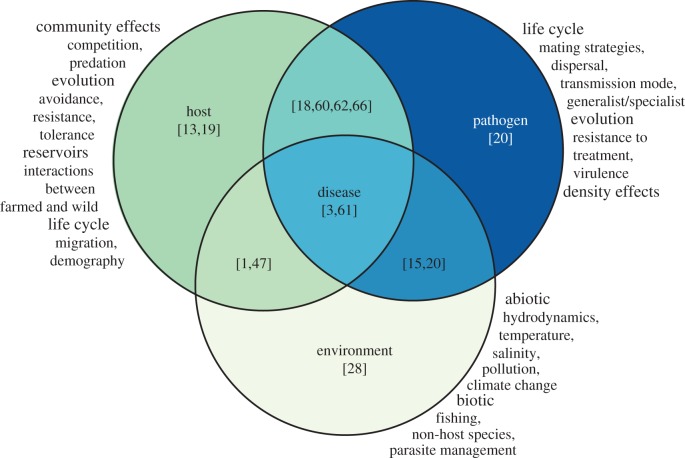
Here, we showcase quantitative methods and advances from the epidemiology of sea lice on wild and farmed salmon hosts (figure 2). We focus on interactions between host salmon, parasitic sea lice and the environment (figure 3) and their relevance to transmission and outbreak dynamics, conservation and the sustainable management of wild fisheries and farmed salmon. As concerns about pathogen-mediated interactions between farmed and wild marine species rise, the lessons learned from sea louse and salmon epidemiology are likely to be increasingly relevant.
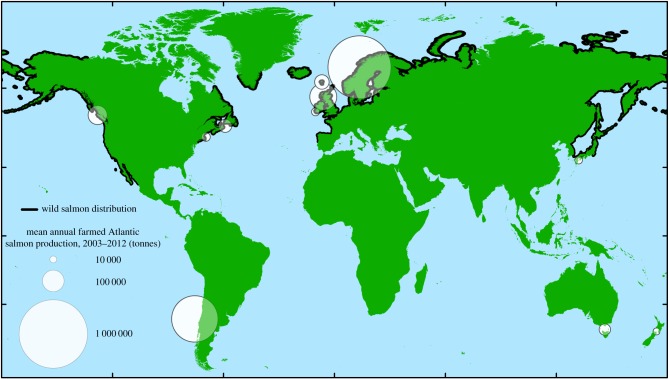
Figure 2.
Global distribution of wild and farmed salmon. Farmed salmon production data were obtained from the Food and Agriculture Organization of the United Nations (FAO) FishStat dataset and include only marine-farmed Atlantic salmon. Countries producing less than 5000 tonnes annually were excluded from the dataset. Circle areas are scaled by mean annual production. Canada is split into two farming regions to differentiate between its Pacific and Atlantic production. For some countries, circles do not encompass all salmon farming locations; in these cases, circles cover the country's region of highest farm density. Wild salmon distribution data were digitized from the FAO's Aquatic Species Distribution Map Viewer, which provides current distributions for Atlantic salmon and the five traditional Pacific salmon species, and modified to exclude waters more than 50 km offshore. (Online version in colour.)
Figure 3.
The development of disease depends on factors of the host, parasite and environment. Studies often focus on one of these components, or the interaction between two, with relatively few studies considering all three components (illustrative references are provided in brackets). (Online version in colour.)
2. Outbreak and transmission dynamics
Understanding when and why outbreaks occur and the means by which they spread is essential for developing a mechanistic understanding of marine diseases. Transmission dynamics in the ocean are variable and complex due to fluctuating water chemistry, oceanographic mixing and, in many cases, free-living infectious stages. This can be further complicated by how aquaculture moves, manages and manipulates host populations.
Most theoretical models assume that increased host population density favours parasite transmission [30]. Indeed, with the exception of vector-borne and sexually transmitted diseases, parasite populations are expected to grow only when hosts exceed a threshold density [31,32]. The presence of host-density dependence in sea louse transmission suggests that an increase in host density can trigger a transition from chronic to acute sea louse outbreak dynamics [33,34]. Statistical models of sea louse population dynamics in Norway [35], stochastic network models of sea louse infection in Loch Fyne, Scotland [36], and hydrodynamic simulations of sea louse production and concentrations in the Broughton Archipelago, British Columbia [28], show how sea louse dynamics can respond to changes in host density among multiple salmon farms and wild salmon populations. Consequently, the relevant spatial scale for critical host-density thresholds in sea louse dynamics, might encompasses several salmon farms, thereby constraining regional production [34].
Temporal variation in host population density can alter epidemiological patterns. Regional host densities in coastal seas experience seasonal pulses of wild salmon biomass. The 1.5- to 2-year seawater production cycles for farmed salmon add additional variability in host density in coastal regions. During spring out-migrations, uninfected juvenile salmon leave their natal streams and estuaries, and migrate through coastal waters to the open ocean. Parametrized advection-diffusion-decay models (table 1) of sea louse dispersal from salmon farms along juvenile salmon migration corridors demonstrate that infection pressure from farm-source sea lice can exceed background (e.g. wild-source) infection pressure in the Broughton Archipelago, British Columbia, Canada [37]. The process of spill-over (from wild to farmed hosts) and spill-back (from farmed to wild hosts) [38] of sea lice is likely initiated by adult wild salmon that carry sea lice into coastal waters from the open ocean during summer and autumn. The natural process of migratory allopatry, where uninfected juvenile salmon are separated from adult salmon and protected from sea lice by migration [39], can be interrupted by the presence of sea louse reservoirs on salmon farms. When these reservoirs were removed by fallowing or parasiticide treatment of farmed salmon, epizootics of sea lice on wild juvenile salmon declined [40], helping to verify model predictions.
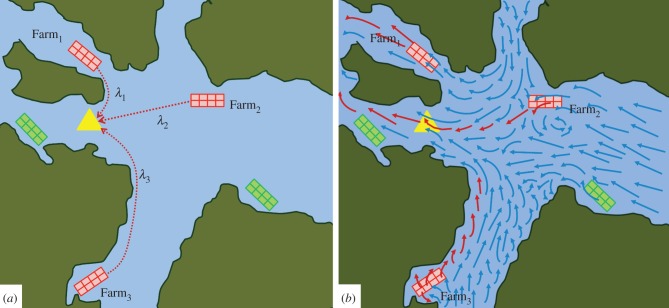
When the density of potential hosts exhibits spatial structure (e.g. from farm placement within oceanographic regions) or spatial movement (e.g. from migrating wild salmon populations), spatially explicit models can improve characterization of parasite transmission. Numerical simulations of ocean currents and wind in the Broughton Archipelago, British Columbia, suggest that hydrodynamic circulation can influence the connectivity between sea louse populations, potentially leading to between-farm source-sink dynamics [28]. By representing sea louse movement as particle flow, these models can account for the influence of water circulation patterns and abiotic properties (e.g. temperature and salinity) on sea lice dispersal. Understanding infective sea lice density and dispersal in the water column is important for identifying farms or regions that are infective sea lice sources and sinks (figure 4). Statistical models have found spatio-temporal correlations between sea louse abundances on farmed and wild salmon up to 30 km apart [41,42]. When coupled with biological models, hydrodynamic simulation models can be used to understand the mechanisms driving these patterns and have been used to characterize transmission of salmon pancreas disease virus among salmon farms in Norway [29], and infectious hematopoietic necrosis virus between salmon farms and wild salmon in Pacific Canada [43]. The contribution of individual farms within a network of farms to regional sea louse connectivity has been quantified using graph theory [36,44] and by merging hydrodynamic modelling of dispersal with classic host-macroparasite models to calculate local and regional thresholds for transmission (table 1) [16].
Figure 4.
Force of infection, λ, exerted from surrounding farms j = 1, 2, 3, on a point location (yellow triangle) can be calculated (a) using distance as a proxy, such that λj is larger for closer farms. In this example, λ1 > λ2 > λ3. In panel (b), λ is calculated more explicitly as per the water flow direction and speed (i.e. longer arrows represent faster flow). Even though farm1 is closest, it is not a likely source of infection given the direction of flow. Further, farm3 is located too far away and the pathogen is not viable by the time it reaches that location of interest. Infection status of farms is shown as dark red (infected) and light green (not infected), and of water carrying viable pathogens as dark red (infected) and light blue (non-viable). (Online version in colour.)
3. Implications for conservation and fisheries
It is important to estimate pathogen impacts before taking management actions. Models can provide the quantitative framework with which to evaluate the evidence for the individual and combined impacts of different stressors (fishing, climate, competition, disease, etc.) on host population size and health. In the case of sea lice, mortality due to disease might be detectable in wild juvenile salmon, which are vulnerable to infections and many other mortality factors, as they migrate to sea [37]. However, if sea louse-induced mortality is compensatory (those that die from infection were likely to have died for other reasons), it will not cause a reduction in adults returning to spawn. Alternatively, if mortality attributable to sea lice is additive to mortality from other causes, such as predation, then overall mortality will increase and could lead to population decline. This was exemplified in a predator–prey–parasite model of infected juvenile pink salmon [5]. The model incorporated a hypothesized saturating predation rate [45] of juvenile pink salmon by juvenile coho salmon and an empirically derived positive relationship between predation on juvenile pink salmon and the infection level of the pink salmon [5]. Analysis of the model suggested that when pink salmon populations were small, infection increased juvenile pink salmon mortality, whereas when they were large, infection and predation were compensatory sources of mortality.
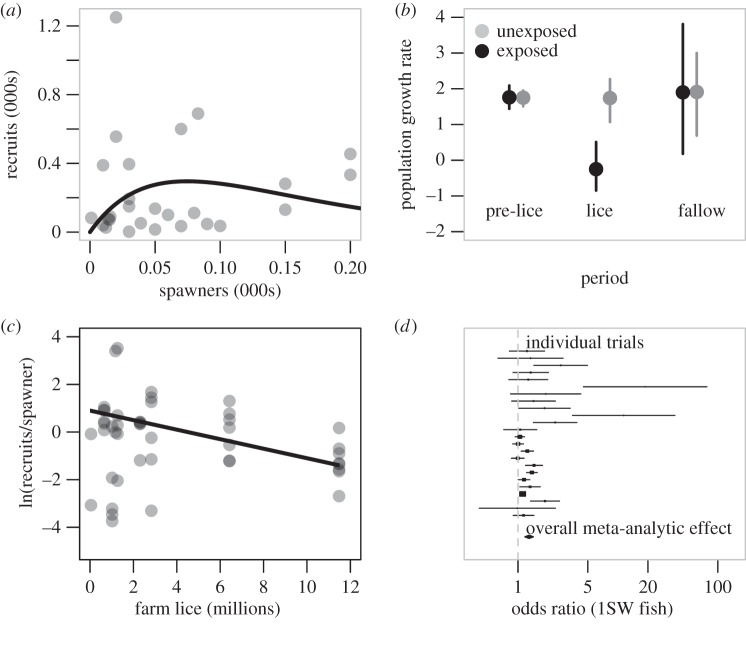
Several studies have evaluated evidence for population-level impacts of sea louse infection on wild salmon. Most studies have been based on models of salmon spawner–recruitment dynamics, which investigate whether sea louse exposure can explain variability in the spawner–recruitment relationship (e.g. [42]) (figure 5a). In spawner–recruitment models, a population's growth rate during sea louse epidemics can be compared to the population growth rate of the same population before or after the epidemic, or to nearby, unexposed salmon populations (figure 5b). Studies on wild pink (Oncorhynchus gorbuscha) [13,24,40] and coho (Oncorhynchus kisutch) [25] salmon in Pacific Canada found evidence that population growth rates are depressed in populations exposed to sea lice outbreaks associated with salmon aquaculture. Similarly, studies on post-smolt wild sea trout (Salmo trutta) in Ireland found that infection levels were higher if the fish were captured in bays that also contained salmon farms [46]. Incorporating information on variability in infection pressure can increase statistical power of such tests (figure 5c). For example, productivity of wild pink and coho salmon has been found to negatively correlate with time-varying regional sea louse abundance on farmed or wild salmon [26,40].
Figure 5.
Stock–recruitment relationships evaluating the effect of sea lice on wild salmon population dynamics. (a) An example Ricker stock–recruitment relationship (the Kwalate coho population in British Columbia) illustrating the observed (grey circles) and predicted (black line) number of individuals in one generation (recruits) produced by spawners in the previous generation. Stock productivity is the maximum ratio of recruits to spawners and is the initial slope of the stock–recruitment curve [25]. (b) Coho salmon population growth rates (±95% bootstrapped CIs) from exposed (black circles) and unexposed regions (grey circles) prior to and during years when salmon lice epidemics were observed in exposed populations as well as during a fallow treatment year [25]. (c) Productivity (loge (recruits per spawner)) of coho salmon populations in relation to the estimated number of lice on farmed fish during juvenile coho seaward migration. The black line is the estimated relationship between productivity and louse abundance based upon a hierarchical linear regression [26]. (d) A random effect meta-analysis of chemotherapeutant treatment on the likelihood of adult Atlantic salmon returning to spawn. Lines are the 95% CIs of the effect size for each trial with the closed diamond representing the overall meta-analytic effect across all studies [3].
Considering multiple populations simultaneously increases the chance that common responses to disease can be separated from other sources of population variability [47]. This is most powerful when salmon populations are used as statistical replicates to identify the underlying ‘signal’ of sea louse exposure, as opposed to single- or aggregate-population level evaluations. For example, analyses seeking to estimate sea louse-associated mortality in pink salmon in Pacific Canada, based on a single aggregate stock–recruitment relationship [48], failed to detect a sea louse signal. However, a signal of sea louse exposure was detected using a multi-population analysis because it had greater statistical power [26]. Observations from individual salmon populations might not be independent if salmon populations are related to a common regional estimate of sea louse infection pressure. In such cases, hierarchical (mixed effects) models can account for the non-independence of responses while simultaneously drawing inferences from many salmon populations (e.g. [26]).
Models can make predictions, but the definitive test for a causal influence of pathogens on host population dynamics requires experimental manipulation of disease in wild host populations. This can be accomplished by experimental reduction of pathogen burdens and the comparison of survival or population dynamics between treated and control groups. Such experiments have been carried out with Atlantic salmon, where thousands of smolts (juvenile salmon) were released in paired trials. Half of the fish were treated with a parasiticide that provided temporary protection from infection by sea lice in early marine life, while the other half received a control treatment. A meta-analysis of 24 paired-release experiments (totalling 283 347 fish) showed that lack of treatment (a surrogate of parasite exposure) decreased survival of returning adults by 39% [3]. Although there was variation among trials in effectiveness of treatments, with some trials showing no evidence of effect and others showing a strong effect, collective examination revealed clear evidence of a positive effect of the parasiticide treatment on salmon recruitment (figure 5d).
4. Management approaches
Direct management of disease is more feasible in farmed systems than in wild populations. As with terrestrial farms, management options range from chemical treatments, to genetic selection for disease resistance, to integrated pest management, to regional coordination of fallowing and stocking. Sea lice management often occurs on a single farm or across several farms within a region. On a farm, fallowing after harvest can interrupt sea louse population growth, while treatments administered to fish can remove or kill attached sea lice [9,10]. Models have been useful for developing management strategies at both levels. Delay-differential equation models have been used to show how two pulsed chemical treatments delivered six weeks apart can be more effective than regular single treatments for controlling the abundance of sea lice [49], while individual-based models that combine chemical treatments with other methods of control (e.g. stocking cleanerfish that eat adult sea lice) are useful for optimizing integrated pest management strategies on farms [27]. Modelling, therefore, can help salmon farmers balance sustainable management practices with economic viability. In particular, coupled biological–economical models of sea louse treatment approaches can help the salmon farm industry conduct more responsive evidence-based management (e.g. [50]).
At a regional scale, generalized linear statistical models indicated that the prevalence of sea lice at the farm level increases with host abundance on that farm and proximity to other infected farms [34,35]. These results support a strategy of coordinated fallowing among farms at spatial and temporal scales chosen to break infection cycling among neighbouring farms. In addition, responsiveness to the connectivity and density among salmon farms, coordination of treatment applications and agreements on treatment thresholds can all help to regulate sea lice at a regional level.
Environmental factors, including water temperature, salinity and water movement, also influence the potential for sea louse epidemics. Modelling these factors might help with forecasting epidemics and siting and stocking salmon farms [51]. In Pacific Canada, a multivariable statistical model of a 10-year dataset of sea louse parasitism on out-migrating chum and pink salmon was made to account for abiotic factors, farm activity and spatial processes of parasite dispersal and fish migration [41]. This comprehensive approach is an invaluable tool for evaluating how marine aquaculture and parasite management policies influence spatial patterns of infection levels on wild fish given environmental conditions [41]. Environmental effects on infections within salmon farms have also been quantified using theoretical simulation models. For example, a modified susceptible-exposed-infective-resistant ordinary differential equation model showed that stronger currents and higher stocking levels increased transmission of sea lice between two hypothetical farms [52]. A matrix model that explicitly accounted for demographic stochasticity among sea lice raised concerns about the impact of rising sea surface temperatures on sea louse dynamics by showing that both high temperatures and increased temperature variation can increase the sea louse basic reproduction number, R0, and decrease generation time of sea lice, resulting in faster population growth [20]. The development of these mathematical models that incorporated environmental impacts has been facilitated by numerous laboratory studies that allowed for the parametrization of stage-specific effects of temperature on sea louse development [53].
Attached sea lice tend to cluster at different spatial scales. This is an important consideration for the design of management strategies or monitoring programmes. As with many parasites, sea lice are often over-dispersed among hosts: a few fish may experience high-intensity infections while most have few or no sea lice [54]. At the farm scale, with multiple cages of salmon, sea lice aggregate more heavily in some cages than in others. At both host and farm scales, this spatial heterogeneity has implications for sea louse demography and monitoring [20]. Monte Carlo simulations and matrix population modelling suggest that when sea louse abundance is maintained below a mean of three gravid females per host on farms, mate limitation decreases the rate of sea louse reproduction [20,55]. However, over-dispersion of sea lice on hosts can increase mating success by bringing males and females into contact more frequently [20]. Over-dispersion may also have disadvantages for parasite fitness, because parasite-induced host mortality can have disproportionate impacts on parasite population dynamics such that each mortality of a heavily infected host provides a substantial reduction in the parasite population [16,54]. From a management perspective, heterogeneity in infection load can hamper estimation of sea louse abundance. Empirical studies and mathematical simulations using individual-based models support the idea that sampling a few fish from many pens, rather than sampling many fish from few pens, improves estimates of louse abundances at the host and farm levels [54]. Because sea louse counts influence management decisions, accurate estimates of infection intensity are essential for optimizing treatment regimens [56]. Therefore, the spatial distribution of sea lice among hosts must be understood and accounted for in monitoring programmes.
Aquaculture practices, particularly chemical treatments on salmon farms, can place selection pressures on sea lice. Despite their implications for sustainable aquaculture, such evolutionary processes are poorly explored in marine disease management [57]. Those studies that exist demonstrate that aquaculture can cause substantial evolution of parasites. For example, microparasites evolved greater virulence when faced with elevated host densities on salmon farms (e.g. [53]) and, in some areas, sea lice have evolved resistance to the chemicals used to control them [58,59]. Ordinary differential equation models and individual-based models show that the frequency of treatments and mixing rates between sea lice on farmed and wild untreated hosts can influence the rate of resistance evolution [60,61]. Mixing between wild-origin sea lice and farm sea lice can dilute the proportion of resistant individuals and provide an opportunity for resistant alleles to be removed by natural selection if they have fitness costs [60].
5. Future directions
Over the past 30 years, substantial progress has been made towards understanding how wild and farmed salmon populations interact to influence infectious disease dynamics. As aquaculture practices change and disease ecology matures as a multidisciplinary field, new applied and theoretical questions continue to emerge. We summarize some of these future directions here.
(a). Co-ordinated management of multiple pathogens
Managing the health of wild and farmed populations extends beyond a single pathogen. When infection with one pathogen increases susceptibility to another (e.g. [62]), or when multiple pathogens may be transmitted through the same hydrodynamic pathways (e.g. [29]), coordinating management of multiple diseases may be advantageous. Coordinated efforts are most effective when they take into account the impacts of disease on the health of the surrounding farms and wild communities and the role that the environment may play in mediating these impacts.
(b). Putting disease into a community context
Interactions between parasites and host communities are numerous [63] and poorly understood for many marine pathogens. Multi-host models are underutilized and could help to improve our understanding of feedback between disease and communities. For example, in both the Pacific and Atlantic Oceans, herring (Clupea sp.) form large potential host populations for generalist sea lice (e.g. C. clemensi and C. elongatus) and differ in their migration timing from co-occurring salmon [64]. The extent to which herring, or other host species, play a significant role in sea louse population dynamics, spread and evolution, is an important, unanswered question.
(c). Effects of aquaculture on evolutionary processes
Despite the progress described above, the evolutionary implications of aquaculture—for hosts and for their parasites—remain under-explored (but see [65]). The potential selection pressures for increased defences in wild hosts and for treatment resistance in pathogens are important considerations for disease management. For sea lice, our understanding of disease-related evolutionary processes is limited to physiological changes in sea lice that make them more tolerant of chemical treatments. However, sea louse behaviour and life history could also undergo rapid selection. Finally, the role of artificial selection for disease-resistant farmed hosts might also influence the dynamics of pathogens on both farmed and wild populations [61].
(d). Potential for better data
Lack of data is a significant barrier to the development of tactical models of many marine diseases. While conceptual models can be parametrized using qualitative trends, site- or situation-specific models require detailed parametrization, and subsequent calibration and validation. In many cases, we lack precise information on the population dynamics of wild populations and their infectious status. Conceptual models might be useful for prioritizing data collection, such as by identifying parameters with high model sensitivity. Ultimately, many management decisions depend upon more specific, well-parametrized tactical models.
The salmon farming industry, government and conservation groups often collect and hold complementary data separately. Some practical advances in data accessibility have already been achieved through collaboration and data sharing. For example, in both Scotland and Atlantic Canada, evidence of sea louse resistance to SLICE® was uncovered through modelling using shared industry-wide datasets well in advance of laboratory-based confirmation [58,59]. Even in the relatively contentious context of Pacific Canada, the Broughton Archipelago Monitoring Plan (www.bamp.ca) has illustrated that progress can be achieved through collaboration among stakeholders from industry, government and non-governmental organizations [15,41,66]. Keeping lines of communication open, while maintaining scientific integrity, will continue to benefit management, science and conservation.
(e). Potential for better statistical models
Greater computing power and more sophisticated statistical methods, now commonplace in ecology, will continue to improve information gained from available data. Where assumptions of simple dynamic patterns and normal errors previously prevailed, advanced Markov chain Monte Carlo methods now allow end-users to fit complex nonlinear, non-normal hierarchical statistical models. These methods are well suited to the study of emerging infectious diseases and we expect to see their use increase.
6. Discussion
Mathematical models are an invaluable tool for understanding complex interactions in sea lice epidemiology. Their role has been to translate hypotheses into quantitative relationships between ecological processes, and they can yield insights into ecological dynamics. Consequently, models are a quantitative framework with which to assess competing claims; a tool to refine, clarify and communicate ideas; and a way to predict the outcomes of ecological or management-based changes. From interpretation of data to development of novel theory, models have both facilitated and driven inquiry.
While many biologists would predict some adverse effects from maintaining elevated densities of host fish, feeding and protecting hosts from predation, and placing farmed hosts in contact with wild migratory hosts, the details of these interactions have not always been clear. Only through the development of relevant hypotheses, translation into models, careful data collection and analysis—and the feedback between them—has progress been possible.
Due in part to conflicts between economics and conservation, there has been disagreement on the nature and magnitude of effects of sea lice on wild populations [13,26,48,67,68]. One major challenge to research has been the inability to undertake ecosystem-wide controlled experiments. This means that, by necessity, manipulative studies are rare compared with correlative studies. Even when manipulative studies have been possible [3,69], debate and controversy have persisted [70,71]. Another major challenge is the fact that a negative statistical result does not necessarily translate to a negative biological conclusion. This second challenge arises because simple lack of evidence is not evidence of lack of an effect, particularly if the statistical power of the approach is low. Fortunately, larger datasets increase statistical power, and a spectrum of ecological conditions gives the possibility of more closely emulating randomized trial conditions found in experimental manipulations.
As the aquaculture industry continues to expand and wild fish populations decline, new pathogens and novel transmission pathways will emerge in both farmed and wild populations, raising similar questions to those asked about sea lice over the past decades. What are the modes of transmission? What is the potential for transmission between wild and farmed fish? If transmitted, how will these pathogens affect individuals and populations? These questions are not unique to salmon and sea lice, and it may be advantageous for scientists, industry and policy-makers to approach other marine infectious diseases in light of all that has been learned in this system. Indeed, in this review, we have outlined only a fraction of the potential interactions among hosts, parasites and their environments. We hope that questions surrounding features and outcomes illustrated here will motivate further study of these complex systems.
Acknowledgements
The authors thank Salmon Coast Field Station for hosting a formative meeting during the early development of the manuscript. Mike Foreman provided useful feedback relating to hydrodynamic modelling. William Chalmers and anonymous reviewers provided thoughtful editorial comments.
Authors' contributions
All authors contributed substantially to the conception and writing of this article.
Competing interests
We have no competing interests
Funding
We acknowledge funding from the following sources: CERC in aquatic epidemiology (M.L.G., E.E.R.); Hakai Doctoral Scholarship and NSERC CGS (L.A.R.); Watershed Watch Salmon Society and NSERC IPS (S.C.G.); NSERC Vanier CGS (S.J.P.); NSERC PDF (A.W.B.); CRC (M.A.L., C.W.R.), NSERC Discovery and Accelerator Grants (M.A.L.) and the Killam Foundation (M.A.L.).
References
- 1.Wood CL, Lafferty KD, Micheli F. 2010. Fishing out marine parasites? Impacts of fishing on rates of parasitism in the ocean. Ecol. Lett. 13, 761–775. ( 10.1111/j.1461-0248.2010.01467.x) [DOI] [PubMed] [Google Scholar]
- 2.Ben-Horin T, Lafferty KD, Bidegain G, Lenihan HS. 2016. Fishing diseased abalone to promote yield and conservation. Phil. Trans. R. Soc. B 371, 20150211 ( 10.1098/rstb.2015.0211) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krkošek M, Revie CW, Gargan PG, Skilbrei OT, Finstad B, Todd CD. 2013. Impact of parasites on salmon recruitment in the Northeast Atlantic Ocean. Proc. R. Soc. B 280, 20122359 ( 10.1098/rspb.2012.2359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvell D, et al. 2004. The rising tide of ocean diseases: unsolved problems and research priorities. Front. Ecol. Environ. 2, 375–382. ( 10.1890/1540-9295(2004)002%5B0375:TRTOOD%5D2.0.CO;2) [DOI] [Google Scholar]
- 5.Krkošek M, et al. 2011. Fish farms, parasites, and predators: implications for salmon population dynamics. Ecol. Appl. 21, 897–914. ( 10.1890/09-1861.1) [DOI] [PubMed] [Google Scholar]
- 6.Godwin S, Dill L, Reynolds J, Krkošek M. 2015. Sea lice, sockeye salmon, and foraging competition: lousy fish are lousy competitors. Can. J. Fish. Aquat. Sci. 72, 1113–1120. ( 10.1139/cjfas-2014-0284) [DOI] [Google Scholar]
- 7.Altizer S, Bartel R, Han BA. 2011. Animal migration and infectious disease risk. Science 331, 296–302. ( 10.1126/science.1194694) [DOI] [PubMed] [Google Scholar]
- 8.Johns S, Shaw A. 2015. Theoretical insight into three disease-related benefits of migration. Popul. Ecol. 58, 213–221. ( 10.1007/s10144-015-0518-x) [DOI] [Google Scholar]
- 9.Pike A, Wadsworth S. 1999. Sealice on salmonids: their biology and control. Adv. Parasitol. 44, 233–337. ( 10.1016/S0065-308X(08)60233-X) [DOI] [PubMed] [Google Scholar]
- 10.Costello MJ. 2006. Ecology of sea lice parasitic on farmed and wild fish. Trends Parasitol. 22, 475–483. ( 10.1016/j.pt.2006.08.006) [DOI] [PubMed] [Google Scholar]
- 11.Krkos˘ek M. 2010. Sea lice and salmon in Pacific Canada: ecology and policy. Front. Ecol. Environ. 8, 201–209. ( 10.1890/080097) [DOI] [Google Scholar]
- 12.Costello MJ. 2009. The global economic cost of sea lice to the salmonid farming industry. J. Fish Dis. 32, 115–118. ( 10.1111/j.1365-2761.2008.01011.x) [DOI] [PubMed] [Google Scholar]
- 13.Krkošek M, Ford J, Morton A, Lele S, Myers RA, Lewis MA. 2007. Declining wild salmon populations in relation to parasites from farm salmon. Science 318, 1772–1775. ( 10.1126/science.1148744) [DOI] [PubMed] [Google Scholar]
- 14.Ricker WE. 1954. Stock and recruitment. Fish. Res. Board Can. 11, 559–623. ( 10.1139/f54-039) [DOI] [Google Scholar]
- 15.Rogers LA, Peacock SJ, McKenzie P, DeDominicis S, Jones SRM, Chandler P, Foreman MGG, Revie CW, Krkošek M. 2013. Modeling parasite dynamics on farmed salmon for precautionary conservation management of wild salmon. PLoS ONE 8, e60096 ( 10.1371/journal.pone.0060096) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson RM, May RM. 1978. Regulation and stability of host-parasite population interactions: I. Regulatory processes. J. Anim. Ecol. 47, 219–247. ( 10.2307/3933) [DOI] [Google Scholar]
- 17.Revie CW, Robbins C, Gettinby G, Kelly L, Treasurer JW. 2005. A mathematical model of the growth of sea lice, Lepeophtheirus salmonis, populations on farmed Atlantic salmon, Salmo salar L., in Scotland and its use in the assessment of treatment strategies. J. Fish Dis. 28, 603–613. ( 10.1111/j.1365-2761.2005.00665.x) [DOI] [PubMed] [Google Scholar]
- 18.Peacock SJ, Connors BM, Krkošek M, Irvine JR, Lewis MA. 2014. Can reduced predation offset negative effects of sea louse parasites on chum salmon? Proc. R. Soc. B 281, 20132913 ( 10.1098/rspb.2013.2913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krkošek M, Lewis MA, Volpe JP. 2005. Transmission dynamics of parasitic sea lice from farm to wild salmon. Proc. R. Soc. B 272, 689–696. ( 10.1098/rspb.2004.3027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groner ML, Gettinby G, Stormoen M, Revie CW, Cox R. 2014. Modelling the impact of temperature-induced life history plasticity and mate limitation on the epidemic potential of a marine ectoparasite. PLoS ONE 9, e88465 ( 10.1371/journal.pone.0088465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Revie CW, Gettinby G, Treasurer JW, Wallace C. 2003. Identifying epidemiological factors affecting sea lice Lepeophtheirus salmonis abundance on Scottish salmon farms using general linear models. Dis. Aquat. Organ. 57, 85–95. ( 10.3354/dao057085) [DOI] [PubMed] [Google Scholar]
- 22.Middlemas SJ, Fryer RJ, Tulett D, Armstrong JD. 2013. Relationship between sea lice levels on sea trout and fish farm activity in western Scotland. Fish. Manag. Ecol. 20, 68–74. ( 10.1111/fme.12010) [DOI] [Google Scholar]
- 23.Connors BM, Juarez-Colunga E, Dill LM. 2008. Effects of varying salinities on Lepeophtheirus salmonis survival on juvenile pink and chum salmon. J. Fish Biol. 72, 1825–1830. ( 10.1111/j.1095-8649.2008.01839.x) [DOI] [Google Scholar]
- 24.Krkošek M, Hilborn R. 2011. Sea lice (Lepeophtheirus salmonis) infestations and the productivity of pink salmon (Oncorhynchus gorbuscha) in the Broughton Archipelago, British Columbia, Canada. Can. J. Fish. Aquat. Sci. 68, 17–29. ( 10.1139/F10-137) [DOI] [Google Scholar]
- 25.Connors BM, Krkošek M, Ford J, Dill LM. 2010. Coho salmon productivity in relation to salmon lice from infected prey and salmon farms. J. Appl. Ecol. 47, 1372–1377. ( 10.1111/j.1365-2664.2010.01889.x) [DOI] [Google Scholar]
- 26.Krkošek M, Connors BM, Morton A, Lewis MA, Dill LM, Hilborn R. 2011. Effects of parasites from salmon farms on productivity of wild salmon. Proc. Natl Acad. Sci. USA 108, 14 700–14 704. ( 10.1073/pnas.1101845108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groner ML, Cox R, Gettinby G, Revie CW. 2013. Use of agent-based modelling to predict benefits of cleaner fish in controlling sea lice, Lepeophtheirus salmonis, infestations on farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 36, 195–208. ( 10.1111/jfd.12017) [DOI] [PubMed] [Google Scholar]
- 28.Stucchi DJ, Guo M, Foreman MGG, Czajko P, Galbraith M, Mackas DDL, Gillibrand PA. 2011. Modeling sea lice production and concentrations in the Broughton Archipelago, British Columbia. In Salmon lice: an integrated approach to understanding parasite abundance and distribution (eds Jones S, Beamish R), pp. 117–150. Chichester, UK: Wiley-Blackwell. [Google Scholar]
- 29.Stene A, Viljugrein H, Yndestad H, Tavornpanich S, Skjerve E. 2014. Transmission dynamics of pancreas disease (PD) in a Norwegian fjord: aspects of water transport, contact networks and infection pressure among salmon farms. J. Fish Dis. 37, 123–134. ( 10.1111/jfd.12090) [DOI] [PubMed] [Google Scholar]
- 30.Arneberg P, Skorping A, Grenfell B, Read AF. 1998. Host densities as determinants of abundance in parasite communities. Proc. R. Soc. Lond. B 265, 1283–1289. ( 10.1098/rspb.1998.0431) [DOI] [Google Scholar]
- 31.Anderson RM, May RM. 1979. Population biology of infectious diseases: part I. Nature 280, 361–367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 32.Lloyd-Smith JO, Cross PC, Briggs CJ, Daugherty M, Getz WM, Latto J, Sanchez MS, Smith AB, Swei A. 2005. Should we expect population thresholds for wildlife disease? Trends Ecol. Evol. 20, 511–519. ( 10.1016/j.tree.2005.07.004) [DOI] [PubMed] [Google Scholar]
- 33.Krkošek M. 2010. Host density thresholds and disease control for fisheries and aquaculture. Aquac. Environ. Interact. 1, 21–32. ( 10.3354/aei0004) [DOI] [Google Scholar]
- 34.Jansen PA, Kristoffersen AB, Viljugrein H, Jimenez D, Aldrin M, Stien A. 2012. Sea lice as a density-dependent constraint to salmonid farming. Proc. R. Soc. B 279, 2330–2338. ( 10.1098/rspb.2012.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aldrin M, Storvik B, Kristoffersen AB, Jansen PA. 2013. Space-time modelling of the spread of salmon lice between and within Norwegian marine salmon farms. PLoS ONE 8, e64039 ( 10.1371/journal.pone.0064039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams T, Black K, MacIntyre C, MacIntyre I, Dean R. 2012. Connectivity modelling and network analysis of sea lice infection in Loch Fyne, west coast of Scotland. Aquac. Environ. Interact. 3, 51–63. ( 10.3354/aei00052) [DOI] [Google Scholar]
- 37.Krkošek M, Lewis MA, Morton A, Frazer LN, Volpe JP. 2006. Epizootics of wild fish induced by farm fish. Proc. Natl Acad. Sci. USA 103, 15 506–15 510. ( 10.1073/pnas.0603525103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 39.Krkošek M, Gottesfeld AS, Proctor B, Rolston D, Carr-Harris C, Lewis MA. 2007. Effects of host migration, diversity and aquaculture on sea lice threats to Pacific salmon populations. Proc. R. Soc. B 274, 3141–3149. ( 10.1098/rspb.2007.1122) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peacock SJ, Krkošek M, Proboszcz S, Orr C, Lewis MA. 2013. Cessation of a salmon decline with control of parasites. Ecol. Appl. 23, 606–620. ( 10.1890/12-0519.1) [DOI] [PubMed] [Google Scholar]
- 41.Rees E, St-Hilaire S, Jones S, Krkošek M, Foreman M, DeDominicis S, Patanasatienkul T, Revie C. 2015. Spatial patterns of parasite infection among wild and captive salmon in western Canada. Landsc. Ecol. 30, 989–1004. ( 10.1007/s10980-015-0188-2) [DOI] [Google Scholar]
- 42.Kristoffersen AB, Rees EE, Stryhn H, Ibarra R, Campisto J-L, Revie CW, St. Hilaire S. 2013. Understanding sources of sea lice for salmon farms in Chile. Prev. Vet. Med. 111, 165–175. ( 10.1016/j.prevetmed.2013.03.015) [DOI] [PubMed] [Google Scholar]
- 43.Foreman MGG, Guo M, Garver KA, Stucchi D, Chandler P, Wan D, Morrison J, Tuele D. 2015. Modelling infectious hematopoietic necrosis virus dispersion from marine salmon farms in the Discovery Islands, British Columbia, Canada. PLoS ONE 10, e0130951 ( 10.1371/journal.pone.0130951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treml EA, Halpin PN, Urban DL, Pratson LF. 2008. Modeling population connectivity by ocean currents, a graph-theoretic approach for marine conservation. Landsc. Ecol. 23, 19–36. ( 10.1007/s10980-007-9138-y) [DOI] [Google Scholar]
- 45.Holling CS. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385–398. ( 10.4039/Ent91385-7) [DOI] [Google Scholar]
- 46.Tully O, Gargan P, Poole W, Whelan K. 1999. Spatial and temporal variation in the infestation of sea trout (Salmo trutta L.) by the caligid copepod Lepeophtheirus salmonis (Krøyer) in relation to soucres of infection in Ireland. Parasitology 119, 41–51. ( 10.1017/S003118209900445X) [DOI] [PubMed] [Google Scholar]
- 47.Myers R, Mertz G. 1998. Reducing uncertainty in the biological basis of fisheries management by meta-analysis of data from many populations: a synthesis. Fish. Res. 37, 51–60. ( 10.1016/S0165-7836(98)00126-X) [DOI] [Google Scholar]
- 48.Marty GD, Saksida SM, Quinn TJ. 2010. Relationship of farm salmon, sea lice, and wild salmon populations. Proc. Natl Acad. Sci. USA 107, 22 599–22 604. ( 10.1073/pnas.1009573108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins C, Gettinby G, Lees F, Baillie M, Wallace C, Revie CW. 2010. Assessing topical treatment interventions on Scottish salmon farms using a sea lice (Lepeophtheirus salmonis) population model. Aquaculture 306, 191–197. ( 10.1016/j.aquaculture.2010.05.006) [DOI] [Google Scholar]
- 50.Hamza K, Rich K, Wheat I. 2014. A system dynamics approach to sea lice control in Norway. Aquac. Econ. Manage. 18, 344–368. ( 10.1080/13657305.2014.959210) [DOI] [Google Scholar]
- 51.Tully O, Poole W, Whelan K, Merigoux S. 1993. Parameters and possible causes of epizootics of Lepeophtheirus salmonis (Krøyer) infesting sea trout (Salmo trutta L.) off the west coast of Ireland. In Pathogens of wild and farmed fish: sea lice (eds Boxshall G, Dafaye D), pp. 202–218. Chichester, UK: CRC Press. [Google Scholar]
- 52.Salama NKG, Murray AG. 2011. Farm size as a factor in hydrodynamic transmission of pathogens in aquaculture fish production. Aquac. Environ. Interact. 2, 61–74. ( 10.3354/aei00030) [DOI] [Google Scholar]
- 53.Stien A, Bjørn PA, Heuch PA, Elston DA. 2005. Population dynamics of salmon lice Lepeophtheirus salmonis on Atlantic salmon and sea trout. Mar. Ecol. Prog. Ser. 290, 263–275. ( 10.3354/meps290263) [DOI] [Google Scholar]
- 54.Revie C, Gettinby G, Treasurer J, Wallace C. 2005. Evaluating the effect of clustering when monitoring the abundance of sea lice populations on farmed Atlantic salmon. J. Fish Biol. 66, 773–783. ( 10.1111/j.0022-1112.2005.00642.x) [DOI] [Google Scholar]
- 55.Stormoen M, Skjerve E, Aunsmo A. 2013. Modelling salmon lice, Lepeophtheirus salmonis, reproduction on farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 36, 25–33. ( 10.1111/j.1365-2761.2012.01415.x) [DOI] [PubMed] [Google Scholar]
- 56.Jimenez DF, Heuch PA, Revie CW, Gettinby G. 2012. Confidence in assessing the effectiveness of bath treatments for the control of sea lice on Norwegian salmon farms. Aquaculture 344–349, 58–65. ( 10.1016/j.aquaculture.2012.03.029) [DOI] [Google Scholar]
- 57.Vander Wal E, Garant D, Calmé S, Chapman CA, Festa-Bianchet M, Millien V, Rioux-Paquette S, Pelletier F. 2014. Applying evolutionary concepts to wildlife disease ecology and management. Evol. Appl. 7, 856–868. ( 10.1111/eva.12168) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lees F, Baillie M, Gettinby G, Revie CW. 2008. The efficacy of emamectin benzoate against infestations of Lepeophtheirus salmonis on farmed Atlantic salmon (Salmo salar L) in Scotland, 2002–2006. PLoS ONE 3, e1549 ( 10.1371/journal.pone.0001549) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones PG, Hammell KL, Gettinby G, Revie CW. 2013. Detection of emamectin benzoate tolerance emergence in different life stages of sea lice, Lepeophtheirus salmonis, on farmed Atlantic salmon, Salmo salar L. J. Fish Dis. 36, 209–220. ( 10.1111/jfd.12022) [DOI] [PubMed] [Google Scholar]
- 60.Murray A. 2011. A simple model to assess selection for treatment-resistant sea lice. Ecol. Modell. 222, 1854–1862. ( 10.1016/j.ecolmodel.2011.03.016) [DOI] [Google Scholar]
- 61.McEwan G, Groner M, Fast M, Gettinby G, Revie C. 2015. Using agent-based modelling to predict the role of wild refugia in the evolution of resistance of sea lice to chemotherapeutants. PLoS ONE 10, e0139128 ( 10.1371/journal.pone.0139128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lhorente JP, Gallardo JA, Villanueva B, Carabaño MJ, Neira R. 2014. Disease resistance in Atlantic salmon (Salmo salar): conifection of the intracellular bacterial pathogen Piscirickettsia salmonis and the sea louse Caligus rogercresseyi. PLoS ONE 9, e95397 ( 10.1371/journal.pone.0095397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hatcher MJ, Dick JT, Dunn AM. 2012. Diverse effects of parasites in ecosystems: linking interdependent processes. Front. Ecol. Environ. 10, 186–194. ( 10.1890/110016) [DOI] [Google Scholar]
- 64.Mackenzie I, Morrison J. 1989. An unusually heavy infestation of herring. Bull. Eur. Ass. Fish Pathol. 9, 12. [Google Scholar]
- 65.Mennerat A, Hamre L, Ebert D, Nilsen F, Dávidová M, Skorping A. 2012. Life history and virulence are linked in the ectoparasitic salmon louse Lepeophtheirus salmonis. J. Evol. Biol. 25, 856–861. ( 10.1111/j.1420-9101.2012.02474.x) [DOI] [PubMed] [Google Scholar]
- 66.Patanasatienkul T, Sanchez J, Rees EE, Krkošek M, Jones SRM, Revie CW. 2013. Sea lice infestations on juvenile chum and pink salmon in the Broughton Archipelago, Canada, from 2003 to 2012. Dis. Aquat. Organ. 105, 149–161. ( 10.3354/dao02616) [DOI] [PubMed] [Google Scholar]
- 67.Riddell B, Beamish R, Richards L, Candy J. 2008. Comment on ‘Declining wild salmon populations in relation to parasites from farm salmon’. Science 322, 1790b ( 10.1126/science.1156341) [DOI] [PubMed] [Google Scholar]
- 68.Krkošek M, Ford JS, Morton A, Lele S, Lewis MA. 2008. Response to comment on ‘Declining wild salmon populations in relation to parasites from farm salmon’. Science 322, 1790c ( 10.1126/science.1156578) [DOI] [PubMed] [Google Scholar]
- 69.Jackson D, Cotter D, Newell J, McEvoy S, O'Donohoe P, Kane F, McDermott T, Kelly S, Drumm A. 2013. Impact of Lepeophtheirus salmonis infestations on migrating Atlantic salmon, Salmo salar L., smolts at eight locations in Ireland with an analysis of lice-induced marine mortality. J. Fish Dis. 36, 273–281. ( 10.1111/jfd.12054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jackson D, Cotter D, Newell J, O'Donohoe P, Kane F, McDermott T, Kelly S, Drumm A. 2014. Response to M Krkošek, C W Revie, B Finstad and C D Todd's comment on Jackson et al. ’Impact of Lepeophtheirus salmonis infestations on migrating Atlantic salmon, Salmo salar L., smolts at eight locations in Ireland with an analysis of lice-induced marine mortality. J. Fish Dis. 37, 419–421. ( 10.1111/jfd.12239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Krkošek M, Revie CW, Finstad B, Todd CD. 2014. Comment on Jackson et al. ‘Impact of Lepeophtheirus salmonis infestations on migrating Atlantic salmon, Salmo salar L., smolts at eight locations in Ireland with an analysis of lice-induced marine mortality’. J. Fish Dis. 37, 415–417. ( 10.1111/jfd.12157) [DOI] [PubMed] [Google Scholar]
- 72.Nylund A, Devold M, Plarre H, Isdal E, Aarseth M. 2003. Emergence and maintenance of infectious salmon anaemia virus (ISAV) in Europe: a new hypothesis. Dis. Aquat. Org. 56, 11–24. ( 10.3354/dao056011) [DOI] [PubMed] [Google Scholar]