Abstract
By accompanying human travels since prehistorical times, the house mouse dispersed widely throughout the world, and colonized many islands. The origin of the travellers determined the phylogenetic source of the insular mice, which encountered diverse ecological and environmental conditions on the various islands. Insular mice are thus an exceptional model to disentangle the relative role of phylogeny, ecology and climate in evolution. Molar shape is known to vary according to phylogeny and to respond to adaptation. Using for the first time a three-dimensional geometric morphometric approach, compared with a classical two-dimensional quantification, the relative effects of size variation, phylogeny, climate and ecology were investigated on molar shape diversity across a variety of islands. Phylogeny emerged as the factor of prime importance in shaping the molar. Changes in competition level, mostly driven by the presence or absence of the wood mouse on the different islands, appeared as the second most important effect. Climate and size differences accounted for slight shape variation. This evidences a balanced role of random differentiation related to history of colonization, and of adaptation possibly related to resource exploitation.
Keywords: insular evolution, three-dimensional geometric morphometrics, first upper molar, house mouse, Mus musculus domesticus
1. Introduction
The relative importance of chance, history and adaptation in evolution is a long-standing issue [1–4]. Stochastic processes are expected to play an important role in fragmented and isolated populations, because of founder effects and drift which are especially relevant in island populations and invasive species [3]. Adaptation is also expected in such contexts because species are encountering new environmental conditions that expose the immigrants to strong selective pressures [5,6]. Accordingly, evolution on islands has been an emblematic model of adaptive evolution since Darwin's finches (e.g. [7]). Genetics and development can further constrain or facilitate evolution along certain directions, for instance due to genetically correlated traits [8]. As these processes intermingle in shaping phenotypes, their respective role is difficult to tease apart [3], a fact that may lead to interpretations of differentiation being a collection of ‘just-so stories’ [9].
The house mouse (Mus musculus) has been accompanying human travels since prehistorical times [10], and as such is one of the ‘best’ worldwide invasives [11]. From the Western European continent, M. musculus domesticus has colonized many remote areas, including islands [12]. Colonization of new environments, climatically and ecologically different from the source should promote adaptive changes especially when the mice meet their physiological limits [13].
Because Western European populations display a complex genetic structure [14,15], island populations will exhibit a variable genetic signature, depending on the colonization source and subsequent demography, which is itself related to human history [16]. Island house mice thus offer a model to investigate the relative importance of population history, adaptation and stochastic events on phenotypic evolution [9].
We addressed this issue by focusing on the differentiation of the first upper molar tooth. Molar teeth have been shown to be influenced by phylogenetic history [17,18] as well as environmental conditions [19,20]. Development may also constrain their evolution [21]. Three-dimensional (3D) geometric morphometrics were used for the first time to quantify tooth shape in several insular populations and continental reference groups. The results were compared with a two-dimensional (2D) analysis comprised of a larger sampling of the same groups. The phylogenetic relationships were assessed based on mtDNA data. The relative effects of allometry, phylogeny, climate and ecology on the morphological differentiation were then investigated.
2. Material and methods
(a). Material
(i). Morphometric sampling
Five hundred and thirty-two mice were part of the 2D morphometric analysis. This set was down-sampled to 90 mice for 3D morphometrics, including only animals with relatively unworn teeth (table 1). The sampling included continental Western Europe and contrasted insular settings (figure 1a): Northern Atlantic (Orkney Archipelago), Macaronesian islands in the sub-tropical mid-Atlantic region (Madeira and Canary Archipelagoes [14,22]), and the sub-Antarctic region (Marion Island; Guillou Island from the Kerguelen Archipelago [23]). All individuals were considered as adults and sub-adults based on the criterion that the third molars were fully erupted, which occurs at weaning. Males and females were pooled as no sexual dimorphism has been documented for tooth morphology in house mice [23,24].
Table 1.
Sampling of the study: group (zone of trapping) and corresponding geographical area, number of first upper molars in the 3D morphometric analysis (N3D) and in the 2D comparison (N2D), number of D-loop sequences (Ngenet), numbers of haplotypes documented (Nhaplo), genetic diversity (Divgenet, within-group p-distance in %).
| group | geographical area | N3D | N2D | Ngenet | Nhaplo | Divgenet |
|---|---|---|---|---|---|---|
| El Hierro | Canary | 8 | 37 | 55 | 8 | 0.1 |
| La Palma | Canary | 3 | 38 | 38 | 10 | 0.1 |
| Tenerife | Canary | 4 | 35 | 48 | 8 | 0.2 |
| Eday | Orkney | 8 | 18 | 11 | 3 | 0 |
| Faray | Orkney | 9 | 12 | 5 | 1 | 0 |
| Papa Westray | Orkney | 7 | 10 | 9 | 2 | 0 |
| Sanday | Orkney | 6 | 8 | 7 | 1 | 0 |
| Guillou 1993 and 2009 | sub-Antartic | 13 | 44 | 79 | 1 | 0 |
| Marion | sub-Antartic | 9 | 92 | 10 | 2 | 0.1 |
| Madeira | Madeira | 12 | 103 | 112 | 32 | 0.3 |
| Col-Bonn | Western Europe | 4 | 14 | 57 | 29 | 0.9 |
| Southern France | Western Europe | 3 | 81 | 71 | 32 | 0.5 |
| Northern Italy | Western Europe | 4 | 40 | 30 | 26 | 0.9 |
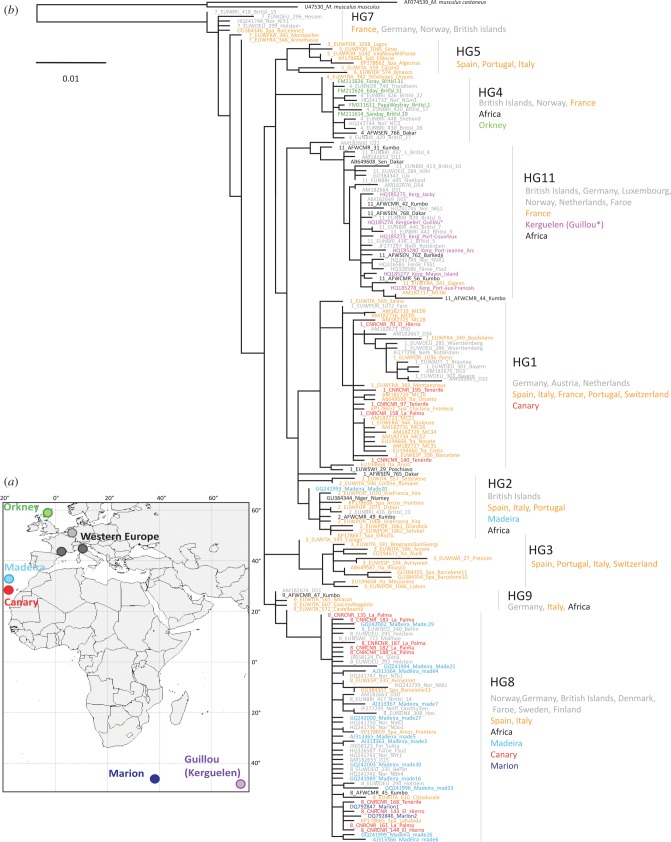
Figure 1.
(a) Map of the localities sampled for morphometrics. (b) Phylogenetic tree based on D-loop sequences. Genetic sampling was designed to encompass at best the diversity of Western European mice, as well as the islands studied. Haplogroups defined by Bonhomme et al. [14] are provided. (Online version in colour.)
(ii). Phylogenetic sampling
Original samples from the Orkney Archipelago were obtained from two field trips in 1992 (islands of Faray, Eday, Sanday and Papa Westray) and 2012 (island of Papa Westray). DNA was extracted from ethanol-preserved tissue of Orkney mice, using the DNeasy Blood and Tissue kit (Qiagen, France). The D-loop was amplified using previously described primers and protocol [25]. The sequences generated were visualized using MEGA6 [26]. No new haplotypes were found in our samples of Orkney populations [16,27]. We combined our sequences with sequences retrieved from GenBank into two datasets. (i) A general dataset to determine the phylogenetic origin of the groups used in the morphometric analysis (electronic supplementary material, table S1). (ii) A dataset designed to include only sequences matching the morphometric sampling (electronic supplementary material table S2). Haplotypes for each group were determined with DNAsp v5 [28] except when this information was already available: Marion Island [29] and Guillou Island [25].
(b). Methods
(i). Phylogenetic analyses
The sequences were aligned with MUSCLE implemented in SeaView [30], the alignment was checked by eye and trimmed at both ends to remove portions with more than 50% of missing data. The final alignments comprised 173 sequences and 947 positions for the general dataset and 155 haplotypes and 874 positions for the morphometric-matching dataset. For this latter dataset, we determined the genetic diversity within each geographical group using MEGA6 [26]. The phylogenetic trees were reconstructed using maximum likelihood with PhyMl v. 3.0 [31] under the models (TN for the general tree and GTR for the morphometric-match, +I + G) selected with jModelTest [32] using the Akaike criterion [33].
(ii). Two-dimensional morphometrics
Using a numerical camera mounted on a binocular, a picture was taken of each mouse molar, with the skull adjusted so that the occlusal surface of the first upper molar would be approximately flat. The molar shape was approximated by the 2D outline of the occlusal surface, towards the base of the crown, which is only affected by heavy wear. Each outline was defined by 64 points, which were analysed using a Fourier-based approach [21]. Fourteen variables, corresponding to a set of Fourier coefficients (FCs) were deemed adequate to describe the molar shape [21]. An additional variable (A0) provided an estimate of the outline size.
(iii). Data acquisition for three-dimensional morphometrics
Skulls were scanned at a cubic voxel resolution of 18 µm using a RX in vivo Skyscan 1076 microtomograph (µCT) device at the Platform Montpellier RIO Imaging. The left first upper molar (UM1) (figure 2) was delimited on each slice using a threshold method in Avizo software (v. 7.1—Visualization Sciences Group, FEI Company) and connections with outer material (jaw bone and second upper molar) were manually closed and the surface generated. On a randomly chosen reference tooth, a template was prepared describing the outer surface of the tooth. As age was not controlled in wild-trapped populations, the template was designed not to take into account parts of the tooth most sensitive to wear: the top of the cusps were cut off the template (electronic supplementary material, figure S1). The template was defined by 1532 equally spaced sliding-landmarks anchored by eight landmarks. These eight landmarks were defined on all specimens and were used for a Procrustes superimposition to align all the specimens in space. Then, the original template was deformed in order to match the original surface of each tooth. Points were allowed to slide along tangent planes according to the minimum bending energy criterion, with an iterative procedure until convergence [34,35]. Sliding-landmarks were adjusted for scaling, translation and rotation according to a Procrustes superimposition. All procedures were performed using the packages ‘Morpho’ [36] and ‘mesheR’ [37]. Procrustes coordinates, i.e. residual coordinates of the sliding-landmarks after Procrustes superimposition, constituted the shape variables describing tooth shape. Centroid size (square root of the sum of the squared distance from each sliding-landmark to the centroid of the configuration) estimated the size of the tooth.
Figure 2.
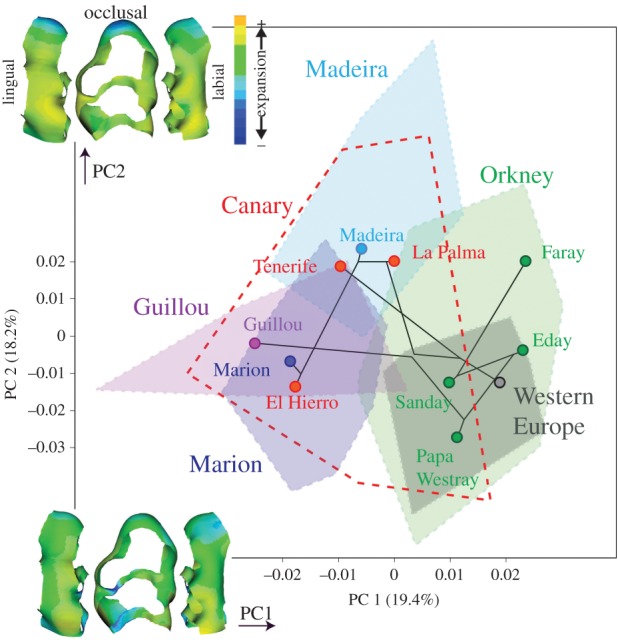
First upper molar differentiation in the morphospace based on 3D morphometrics. Symbols are group means linked by the phylogenetic relationship based on D-loop distances. Envelopes depict range of variation of the geographical groups. Depicted shape changes: along PC1 (from −0.06 to 0.04) and PC2 (from −0.04 to 0.06). (Online version in colour.)
(iv). Statistical analyses of tooth size and shape
Based on 3D morphometric data, differences in tooth size were investigated by an analysis of variance. Allometric variation was assessed using a multivariate regression on the Procrustes coordinates. The residuals were considered as new allometry-free variables. A principal component analysis (PCA) on the size-free variables provided a representation of morphometric variation.
(v). Projection of the phylogeny on the morphometric space
A matrix of genetic distances based on D-loop haplotypes of sequences from the same geographical areas as the morphometric samples, without outgroups, was designed (electronic supplementary material, table S2). It was analysed using a principal coordinate analysis (PCOA) [38] using the R ‘ape’ package [39]. It converted the distance matrix into coordinates on principal axes. Mean values for each group were computed and compared with the morphometrics mean values. The phylogenetic relationships were projected on the morphometric space using ‘phytools’ R package [40], the ancestral states being calculated at each node using ‘fastAnc’ function.
(vi). Size, phylogeny, climate and ecology as explanatory variables of morphometric variation
A linear model was used to investigate the effects of size, phylogeny, ecology and climate on tooth shape. (i) Size was evaluated as the centroid size of the tooth. (ii) Phylogeny was included as the first four axes of the PCOA on the D-loop distance matrix, including the set of axes with greater than 90% of variance. (iii) Climatic data were extracted from the WorldClim database with a resolution of 2.5 arc-min using the ‘raster’ package [41]. The data included annual mean temperature, temperature seasonality, mean warmest quarter, mean coldest quarter, annual precipitation, precipitation of the wettest quarter, precipitation of the driest quarter. A PCA was performed to summarize these partly redundant data. The first three PCs explaining greater than 90% of the total variance were retained as explanatory variables in the model. (iv) Ecological coding included the presence/absence of competitors and of predators according to the literature (electronic supplementary material, table S3) and coded these data as 0/1 (table 1; electronic supplementary material, table S4). As house mice strongly rely on human populations for resources and transport, human population density was also included as an explanatory variable.
Finally, the residuals of this model were analysed using a between-group and within-group PCA using the ade4 package [42]. This procedure allowed us to assess the percentage of variance attributed to between- versus within-group variance in the residual shape variation.
(vii). Comparison between three- and two-dimensional morphometrics
A PCA was performed on the FCs of the 2D outline. The scores of the group means on PC axes provided a configuration that was compared with that of the group means obtained by the 3D approach using a Procrustes superimposition procedure (Protest [43]). The significance of the association was tested using permutations. Distances between the two configurations were further compared using a Mantel test. The linear model of shape (PC axes) versus size (A0), phylogeny, ecology and climate (same variables as for the 3D analysis) was further used on the 2D data in order to investigate the stability of the results to method and sampling.
Visualizations of shape changes were performed using the ‘Morpho’ package [36]. PCOA and Protests were performed using the ‘vegan’ package [44]. Three-dimensional surfaces of the teeth are available for download at MorphoSource.org, under the name ‘Bigtooth—mouse tooth project’.
3. Results
(a). Phylogeny
The continental Western European groups displayed a large haplotypic diversity. Each island represented a subsampling of this diversity (table 1). The founding of the insular populations appears to be so recent regarding the evolutionary rate of the genetic marker that no island displayed a unique haplotype, hindering the estimate of a divergence date. Four independent instances of insular colonization could be identified, in agreement with previous studies (figure 1b). (i) Orkney nested into a mostly Scandinavian and British haplogroup [16], which has been interpreted as the signature of a Norwegian Viking colonization. (ii) Guillou shared its only haplotype with other mice from Kerguelen Archipelago and Western European specimens from England, Germany and France, and from harbours on the way to the Southern Ocean as in Cameroon [25]. (iii) Madeira, the Canary islands of La Palma and El Hierro, and Marion Island were mostly nested within a Northern European haplogroup [14]. This genetic assignation has been interpreted as related to a possible introduction by Danish Vikings onto Madeira [45], mice being later translocated to the Canaries by Portuguese travels. (iv) Tenerife appeared more related to a Southern European haplogroup, a signature of exchanges between the Canaries and the Spanish realms [14]. Evidence of mixing exist on Madeira and all three Canary islands investigated (figure 1; electronic supplementary material, figure S2): typical Tenerife haplotypes seldom occur on Madeira, El Hierro and La Palma, and vice versa.
(b). Three-dimensional tooth morphology
Tooth size varied significantly across populations (p < 0.001; electronic supplementary material, figure S3). Insular mice tended to display larger molars than their continental relatives.
The size–shape allometric relationship was significant (p < 0.001). The analysis of allometry-free residuals provided two axes of almost equal importance (19% and 18%) along which a geographical structure emerged (figure 2). Western European continental teeth clustered together, whereas insular teeth by far exceeded this continental range of variation. Changes along PC1, mostly corresponding to the transition from continental Western European to Guillou–Marion–El Hierro morphologies, involved a pinching at the labial forepart and deepening of the lingual gutter between the central and lingual rows of cusps. Along PC2, characterizing the Macaronesian Madeira–La Palma group, the tooth mostly shortened in its forepart and broadened laterally.
The morphometric structure partly reflected the phylogenetic relationships, with obvious discrepancies. The different Orkney islands clustered together but they displayed an important variation constrasting with their genetic homogeneity. Macaronesian islands from Madeira, Tenerife and La Palma were grouped together, a geographical cluster contradicting the distinct haplotypic dominant signature of Tenerife. Marion and El Hierro, genetically close to the La Palma–Madeira group, were morphologically well differentiated. Guillou Island displayed a convergence in molar shape with Marion and El Hierro, despite a different genetic/geographical origin.
The 2D analysis (electronic supplementary material, figure S4) provided a correlated configuration of between-group differentiation (comparison between PCs > 5% (5 in 3D and 5 in 2D): Protest p = 0.006, Mantel p = 0.008). As in 3D, Western European samples appeared well clustered. The convergence between Tenerife, La Palma and Madeira, on the one hand, and between Guillou Island and El Hierro, on the other hand, were further supported. The idiosyncrasy of Orkney, by which all islands grouped together in the 3D analysis, was not captured by the 2D outline. Orkney islands appeared as widely dispersed in the corresponding morphospace.
(c). Tooth shape versus size, phylogeny, climate and ecology
The total shape variation of the tooth could be summarized along five axes, totalling more than 60% of variance (19.1%, 18.6%, 10.9%, 6.1% and 5.7%). Climatic data were summarized on three PC axes (66.4%, 19.3% and 13.1% of variance). Phylogenetic data were summarized on four PCOA axes (58.0%, 20.7%, 9.9% and 3.7%). Ecological data (competition, predation, human density) were further included in the linear model.
The model indicated a weak contribution of size (3.4%), a balanced influence of ecology (7.3%) and climate (6.0%), and the strongest influence of phylogeny (12.1%) (all p < 0.01, competition and phylogeny p < 0.001) (figure 3). Effects on tooth shape were the following. (i) Size: larger molars were longer at their forepart and thinner in their labial region. (ii) Phylogeny: the first axis, roughly opposing continental Western Europe to Orkney and Macaronesian islands, corresponded to an anterior elongation and a reduction of the protocone and neighbouring lingual cusp. (iii) Ecology: among competition, predation and human density, only competition had a significant effect. Decrease in competition involved a forepart expansion together with an overall thinning of the cusps. (iv) Climate: with a temperature decrease and a precipitation increase (from Macaronesian to sub-Antarctic islands), central cusps moved forward and the anterior lingual fringe expanded. From seasonal (continental) to less seasonal (more or less all islands) environments, the tooth lenghthened in its forepart and most cusps shortened. Adding more axes (e.g. 11 axes, totalling more than 80% of variance) does not change the order of the explanatory factors, and seems instead to add noise and decrease the variance explained by the model: phylogeny (9.4%), ecology (7.5%), climate (5.7%) and size (2.4%). Similar results were found based on the 2D outline analysis. Compared with the 3D, the 2D analysis relied on less shape variables and hence, less axes were required to explain as much morphometric variance, the five first axes totalling 87% of variance. The order of the explanatory factors was retained but the model explained less variance than for 3D: phylogeny (7.2%), ecology (3.5%), climate (2.7%) and size (1.0%). The 2D sampling includes teeth of all wear stage, which may lead to uncertainty in the orientation of the occlusal plane and possibly explain the larger percentage of unexplained variance.
Figure 3.
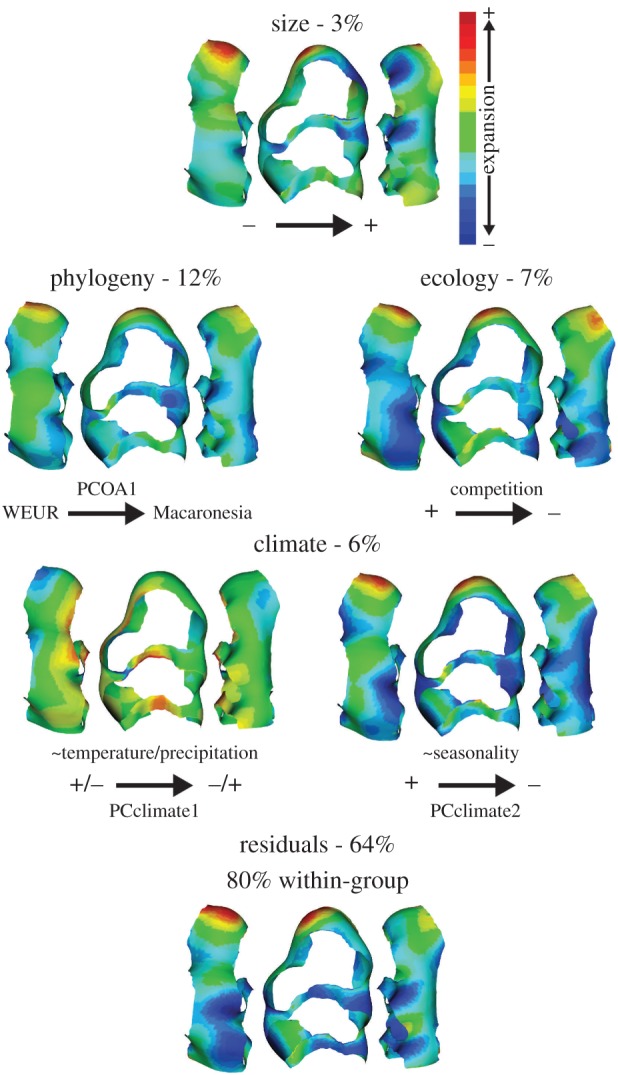
Summary of a model of 3D tooth shape versus explanatory variables: size, phylogeny, ecology and climate and visualization of the various effects. Allometry: shape change with a size increase from 5% to 95% of the distribution. The following representations were computed based on the 29 first PCs (totaling more than 95% of variance) on the size-free variables. Phylogeny: changes along the first phylogenetic axis, roughly corresponding to changes from Western Europe to the Macaronesian cluster. Ecology: change from the presence to absence of inter-specific competition. Climate: changes along the first climatic axis (opposing warm, dry to cold, wet environments) and along the second climatic axis (opposing seasonal, continental environments to non-seasonal, insular environments). In all cases, the shape change between 5% and 95% of the distribution is visualized. The residuals of the model including size, phylogeny, climate and ecology were decomposed into between-group and within-group variances. Shape changes along the first axis of within-group variance are depicted (±0.4 along wgPC1). (Online version in colour.)
Removing size, phylogeny, climate and ecology provided 64.4% residual variance. This residual variation corresponded mostly (80.1%) to within-group variance. The remaining within-group variance corresponded to a trend of anterior expansion combined with a backward movement of the main cusps and their overall thinning. A similar percentage of remaining within-group variance was found in 2D (79.1%).
4. Discussion
(a). Methodological originality: tooth evolution in three dimensions
This study is the first one to document tooth shape variation of house mice in its 3D complexity. It largely corroborated results obtained by 2D analyses, evidencing the importance of anterior tooth elongation in the evolution of the mouse upper molar [21,46]. It further illustrated complex changes in the shape of the cusps and their relative position, up to the deepening of gutters (sulci) between rows of cusps. By describing such features, the 3D description appeared as more efficient than the 2D in assessing the idiosyncrasy of the Orkney Archipelago. The possible functional significance of such changes is unexplored, as such shape changes have not been described so far.
(b). Phylogenetic history as a key factor in tooth shape evolution
The phylogenetic signal appeared of primary importance in explaining first upper molar shape. We focused on this molar because of its high taxonomic value within murine rodents [47]. Within M. musculus, a strong imprint of historical factors is well documented on the first lower molar shape, which is used as a valuable proxy for identifying subspecies [18] and even for tracing the geographical origin within a subspecies [17,18,24]. As covariation of the occluding lower and upper molars is under functional constraints [48], a phylogenetic signal may also be expected on the upper molar. However, the first upper molar was shown to be more evolvable than its lower counterpart [21]. Our present results demonstrate that high evolvability of the upper molar does not fully override the signature of the history of colonization on molar shape.
Discrepancies between the morphological and phylogenetic signature raised questions regarding population history. Madeira, La Palma and Tenerife shared a similar tooth shape. The morphological similarity of Madeira and La Palma was expected given their phylogenetic relatedness [14], possibly reflecting early trading routes between the Madeira and Canary Archipelagoes by the Portuguese during the fifteenth century. The morphological similarity between Tenerife and La Palma was not surprising given their geographical proximity, but was in conflict with the difference in the dominant mitochondrial haplogroup in both islands. The sporadic occurrence of Tenerife-like haplotypes on La Palma, and La Palma-like haplotypes on Tenerife, suggested that gene flow occurred between the neighbouring islands and that the resilience of local populations to later invaders [25] may break down when human exchanges are important. The similar tooth morphology on Tenerife and La Palma may be due to a genetic homogenization on the Canary Archipelago that would not be traced by mtDNA, argued to be a signature of the initial colonization [16,25]. Alternatively, it could be a convergence in similar ecological and climatic environments. The persistence of this tooth shape despite multiple colonizations anyway suggests a resilience of this morphology and/or strong selective pressures maintaining it in the insular populations.
The morphological cluster associating Guillou, Marion and El Hierro teeth constituted another discrepancy with phylogeny. (i) Marion and El Hierro were genetically associated with La Palma–Madeira based on their dominant haplotypes. (ii) The Guillou population derived from an independent colonization event with a different phylogenetic signature. This demonstrated that factors other than phylogenetic history (as traced by mitochondrial markers) contributed to the divergence in molar shape.
(c). Competition as a driving evolutionary force on molar shape
Ecological factors, predominantly inter-specific competition, emerged as driving forces almost as important as phylogeny in explaining molar shape divergence. Differences in competition levels are mostly related to the occurrence of the wood mouse Apodemus sylvaticus on the Western European continent and most Orkney islands, and its absence on Macaronesian archipelagoes and in sub-Antarctic islands (table 1; electronic supplementary material, data S3). The wood mouse is a competitor of the house mouse, limiting its presence in non-commensal habitats where it occurs [49]. In the absence of the wood mouse, the house mouse may exploit more outdoor resources instead of remaining strictly commensal [50]. The tooth shape changes associated with the absence of the wood mouse corresponds to the anterior elongation. Narrow, elongated teeth have been associated in murine rodents with a rather faunivorous diet [51]. Considering this diet/tooth shape trend, non-commensal house mice may be relying more on invertebrates in the absence of the wood mouse, especially when resources are scarce as on sub-Antarctic islands [52,53].
(d). Secondary importance of climate
In addition to phylogeny and ecology, climate further impacted tooth shape. Temperature and precipitation mainly opposed warm, dry Macaronesian islands to cold, wet Orkney and sub-Antarctic islands. The climatic regime changes the available resources and thus constitutes an indirect selective pressure on tooth shape. For instance, mice on the barren sub-Antarctic Marion and Guillou islands are known to increase the invertebrate component in their diet [52,54]. However, the exploited resources also depend on variation in the commensal way of life. On Madeira and Canary islands, mice rely on outdoor resources of anthropic origin, e.g. gardens [55], whereas they were trapped indoors in most continental and Orkney locations (except Faray). Such effects are difficult to quantify and may indirectly impact our results through climate or ecology.
(e). Insularity magnifying phylogenetic and adaptive differences
The pattern of genetic diversity, high on the continent (within-group p-distances: 0.5–0.9%) and low on islands (0–0.3%) contrasted with the pattern of tooth shape differentiation, showing a low variance among continental specimens but a large differentiation for insular samples. This differentiation occurred quite rapidly, from approximately 1200 years for Orkney, most probably colonized following Viking routes [16] to less than 200 years for sub-Antarctic islands where mice were brought by sealers during the nineteenth century [56,57]. Stochastic events are reputed important on islands, with founder events and drift in populations of reduced effective size. Such factors likely promoted the important and rapid divergence from the continental stock, matching a general observation of fast initial divergence upon arrival on an island [58,59]. The importance of the geographical origin [9] is underlined here by the phylogenetic imprint on tooth morphology. Subsequent divergence occurred under constraints related to the local environment: when phylogeny, ecology and climate are taken into account, only approximately 20% of between-group variance remains unexplained. Note that a rather similar hierarchy of factors and percentage of variance were found in the divergence between species of marmots [60] suggesting both the generality of this trend (phylogeny explaining slightly more than climate in tooth divergence) and the importance of divergence occurring at the intra-specific level in insular house mice.
(f). Repeated tooth elongation: a line of least evolutionary resistance?
Anterior tooth elongation appeared as a recurrent feature of shape variation, involved in the response to size increase, to competition and to seasonality. It also corresponded to the residual within-group variation, matching previous 2D observations [21,61]. The main direction of within-group variance has been suggested to constitute a ‘line of least resistance to evolution’ [62] producing variants to be screened by selection. The recurrent mobilization of the anterior tooth shape elongation may document the existence of a standing variation for this trait, explaining its potential for fast and convergent evolution. By contrast, phylogenetic signatures seemed to involve much more localized and discrete morphological features, suggesting that such changes may simply accumulate at a low pace [63]. Our 3D results point out that, contrary to predictions [64], substantial evolution and adaptation can occur even when accounting for the whole complexity of a phenotype. However, signals of convergent evolution and fast divergence appear even stronger when using a simplified description of the tooth, namely its 2D quantification describing only the overall arrangement of the cusps. These are challenging results suggesting that as one of the oldest passengers of human travels, one of the best worldwide invasives, and one of the most studied laboratory models in developmental biology, the house mouse offers a unique opportunity to unravel the complexity of adaptation.
Supplementary Material
Acknowledgements
We thank Renaud Lebrun (ISEM, Montpellier) for his contribution in managing CT scans, produced within the technical facilities of the MRI platform and of the labEx CeMEB. We also thank Jean-Michel Gaillard (LBBE, Lyon) for stimulating discussions, Anne-Béatrice Dufour and Stephane Dray (LBBE, Lyon) for valuable advice on statistical issues and R packages. Josette Catalan, Annie Orth, Sylvie Agret and Lionel Hautier (ISEM, Montpellier) are acknowledged for their participation to the Orkney field trip in 2012. The constructive remarks of P. David Polly and an anonymous reviewer were greatly appreciated. This is publication ISEM 2016-001.
Ethics
Orkney mice were obtained with authorization n° CEEA-LR-12162 from the Languedoc-Roussillon Comité d'Ethique pour l'Expérimentation Animale to J.C.A. Other samples were from pre-existing collections and were previously published. The sacrifice of wild animals for the purpose of taking samples, when performed according to authorized protocols, is not considered as an experiment (Journal Officiel de la République Française, Décret n° 2013-118 du 1er février 2013, Section 6, Sous-section 1). As such, agreement of the ethical committees is not required.
Data accessibility
No new sequences were deposited, as the newly generated sequences all corresponded to sequences already available in GenBank. The dataset including morphometric, climatic, ecologic and genetic data is available as the electronic supplementary material.
Authors' contributions
S.R., R.L., P.C., J.B.D. and J.C.A. conceived and designed the experiments. J.C.A., P.C., G.G., J.B.D., J.L.C., B.P., M.L.M., E.A.H. and S.R. participated in the field trips and/or delivered samples. R.L. and S.S. performed the 3D morphometrics and associated statistics. P.C. undertook the phylogenetic analyses. R.L., P.C. and S.R. prepared the illustrations. All authors contributed in writing the main text.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the ANR project Bigtooth (ANR-11-BSV7-008) and the French Polar Institute (IPEV programme 136).
References
- 1.Pergams ORW, Ashley MV. 2001. Microevolution in island rodents. Genetica 112–113, 245–256. ( 10.1023/A:1013343923155) [DOI] [PubMed] [Google Scholar]
- 2.Travisano M, Mongold JA, Bennet AF, Lenski RE. 1995. Experimental tests of the roles of adaptation, chance, and history in evolution. Science 267, 87–90. ( 10.1126/science.7809610) [DOI] [PubMed] [Google Scholar]
- 3.Keller SR, Taylor DR. 2008. History, chance and adaptation during biological invasion: separating stochastic phenotypic evolution from response to selection. Ecol. Lett. 11, 852–866. ( 10.1111/j.1461-0248.2008.01188.x) [DOI] [PubMed] [Google Scholar]
- 4.Berry RJ. 1973. Chance and change in British long-tailed field mice (Apodemus sylvaticus). J. Zool. 170, 351–366. ( 10.1111/j.1469-7998.1973.tb01383.x) [DOI] [Google Scholar]
- 5.Carroll SP. 2008. Facing change: forms and foundations of contemporary adaptation to biotic invasions. Mol. Ecol. 17, 361–372. ( 10.1111/j.1365-294X.2007.03484.x) [DOI] [PubMed] [Google Scholar]
- 6.Losos JB, Schoener TW, Warheit KI, Creer D. 2001. Experimental studies of adaptive differentiation in Bahamian Anolis lizards. Genetica 112–113, 399–415. ( 10.1023/A:1013387705408) [DOI] [PubMed] [Google Scholar]
- 7.Losos JB, Ricklefs RE. 2009. Adaptation and diversification on islands. Nature 457, 830–836. ( 10.1038/nature07893) [DOI] [PubMed] [Google Scholar]
- 8.Beldade P, Koops K, Brakefield PM. 2002. Developmental constraints versus flexibility in morphological evolution. Nature 416, 844–847. ( 10.1038/416844a) [DOI] [PubMed] [Google Scholar]
- 9.Berry RJ. 1996. Small mammal differentiation on islands. Phil. Trans. R. Soc. Lond. B 351, 753–764. ( 10.1098/rstb.1996.0070) [DOI] [PubMed] [Google Scholar]
- 10.Vigne J-D, Zazzo A, Thomas C, Guilaine J. 2014. The transportation of mammals sheds light on early voyaging and boats in the Mediterranean Sea. Eurasian Prehistory 10, 157–176. [Google Scholar]
- 11.Lowe S, Browne M, Boudjelas S, De Poorter M. 2000. 100 of the world's worst invasive alien species. A selection from the Global Invasive Species Database, pp. 1–12. Auckland, New Zealand: The Invasive Species Specialist Group. [Google Scholar]
- 12.Guénet J-L, Bonhomme F. 2003. Wild mice: an ever-increasing contribution to a popular mammalian model. Trends Genet. 19, 24–31. ( 10.1016/S0168-9525(02)00007-0) [DOI] [PubMed] [Google Scholar]
- 13.Berry RJ, Peters J, Van Aarde RJ. 1978. Sub-Antarctic house mice: colonization, survival and selection. J. Zool. 184, 127–141. ( 10.1111/j.1469-7998.1978.tb03270.x) [DOI] [Google Scholar]
- 14.Bonhomme F, Orth A, Cucchi T, Rajabi-Maham H, Catalan J, Boursot P, Auffray J-C, Britton-Davidian J. 2011. Genetic differentiation of the house mouse around the Mediterranean basin: matrilineal footprints of early and late colonization. Proc. R. Soc. B 278, 1034–1043. ( 10.1098/rspb.2010.1228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardouin EA, Orth A, Teschke M, Darvish J, Tautz D, Bonhomme F. 2015. Eurasian house mouse (Mus musculus L.) differentiation at microsatellite loci identifies the Iranian plateau as a phylogeographic hotspot. BMC Evol. Biol. 15, 26 ( 10.1186/s12862-015-0306-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Searle JB, et al. 2009. Of mice and (Viking?) men: phylogeography of British and Irish house mice. Proc. R. Soc. B 276, 201–207. ( 10.1098/rspb.2008.0958). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cucchi T. 2008. Uluburun shipwreck stowaway house mouse: molar shape analysis and indirect clues about the vessel's last journey. J. Archaeol. Sci. 35, 2953–2959. ( 10.1016/j.jas.2008.06.016) [DOI] [Google Scholar]
- 18.Cucchi T, Bălăşescu A, Bem C, Radu V, Vigne J-D, Tresset A. 2011. New insights into the invasive process of the eastern house mouse (Mus musculus musculus): evidence from the burnt houses of Chalcolithic Romania. Holocene 21, 1195–1202. ( 10.1177/0959683611405233) [DOI] [Google Scholar]
- 19.Piras P, Marcolini F, Raia P, Curcio MT, Kotsakis T. 2009. Testing evolutionary stasis and trends in first lower molar shape of extinct Italian populations of Terricola savii (Arvicolidae, Rodentia) by means of geometric morphometrics. J. Evol. Biol. 22, 179–191. ( 10.1111/j.1420-9101.2008.01632.x) [DOI] [PubMed] [Google Scholar]
- 20.Wolf M, Friggens M, Salazar-Bravo J.. 2009. Does weather shape rodents? Climate related changes in morphology of two heteromyid species. Naturwissenschaften 96, 93–101. ( 10.1007/s00114-008-0456-y) [DOI] [PubMed] [Google Scholar]
- 21.Renaud S, Pantalacci S, Auffray J-C. 2011. Differential evolvability along lines of least resistance of upper and lower molars in island house mice. PLoS One 6, e18951 ( 10.1371/journal.pone.0018951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Britton-Davidian J, Catalan J, Ramalhinho MDG, Auffray J-C, Nunes AC, Gazave E, Searle JB, Mathias MDL. 2005. Chromosomal phylogeny of Robertsonian races of the house mouse on the island of Madeira: testing between alternative mutational processes. Genet. Res. 86, 171–183. ( 10.1017/S0016672305007809) [DOI] [PubMed] [Google Scholar]
- 23.Renaud S, Hardouin EA, Pisanu B, Chapuis J-L. 2013. Invasive house mice facing a changing environment on the Sub-Antarctic Guillou Island (Kerguelen Archipelago). J. Evol. Biol. 26, 612–624. ( 10.1111/jeb.12079) [DOI] [PubMed] [Google Scholar]
- 24.Valenzuela-Lamas S, Baylac M, Cucchi T, Vigne J-D. 2011. House mouse dispersal in Iron Age Spain: a geometric morphometrics appraisal. Biol. J. Linn. Soc. 102, 483–497. ( 10.1111/j.1095-8312.2010.01603.x) [DOI] [Google Scholar]
- 25.Hardouin E, Chapuis J-L, Stevens MI, van Vuuren JB, Quillfeldt P, Scavetta RJ, Teschke M, Tautz D. 2010. House mouse colonization patterns on the sub-Antarctic Kerguelen Archipelago suggest singular primary invasions and resilience against re-invasion. BMC Evol. Biol. 10, 325 ( 10.1186/1471-2148-10-325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S.. 2013. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729. ( 10.1093/molbev/mst197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nachman MW, Boyer SN, Searle JB, Aquadro CF. 1994. Mitochondrial DNA variation and the evolution of Robertsonian chromosomal races of house mice, Mus domesticus. Genetics 136, 1105–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Librado P, Rozas J. 2009. DnaSP v5: a software for comprehensive analysis of DNA polymorphism data. Bioinformatics 25, 1451–1452. ( 10.1093/bioinformatics/btp187) [DOI] [PubMed] [Google Scholar]
- 29.van Vuuren BJ, Chown SL. 2007. Genetic evidence confirms the origin of the house mouse on sub-Antarctic Marion Island. Polar Biol. 30, 327–332. ( 10.1007/s00300-006-0188-4) [DOI] [Google Scholar]
- 30.Gouy M, Guindon S, Gascuel O, Lyon D.. 2010. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224. ( 10.1093/molbev/msp259) [DOI] [PubMed] [Google Scholar]
- 31.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. ( 10.1093/sysbio/syq010) [DOI] [PubMed] [Google Scholar]
- 32.Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 ( 10.1038/nmeth.2109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akaike H. 1973. Information theory as an extension of the maximum likelihood principle. In Second Int. Symp. on Information Theory (eds Petrov BN, Csaki F), pp. 267–281. Budapest, Hungary: Academiai Kiado. [Google Scholar]
- 34.Bookstein FL. 1997. Landmark methods for forms without landmarks: morphometrics of group differences in outline shape. Med. Image Anal. 1, 225–243. ( 10.1016/S1361-8415(97)85012-8) [DOI] [PubMed] [Google Scholar]
- 35.Gunz P, Mitteroecker P, Bookstein FL. 2005. Semilandmarks in three dimensions. Modern morphometrics in physical anthropology. In Modern morphometrics in physical anthropology (ed. Slice DE.), pp. 73–98. Berlin, Germany: Springer. [Google Scholar]
- 36.Schlager S. 2014. Morpho: calculations and visualizations related to geometric morphometrics. R package, 2.0.0.140402 ed. See https://cran.r-project.org/web/packages/Morpho/index.html.
- 37.Schlager S.2013. mesheR: meshing operations on triangular meshes. R package version 0.4-00. See https://github.com/zarquon42b/mesheR .
- 38.Gower JC. 1966. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53, 325–338. ( 10.1093/biomet/53.3-4.325) [DOI] [Google Scholar]
- 39.Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290. ( 10.1093/bioinformatics/btg412) [DOI] [PubMed] [Google Scholar]
- 40.Revell LJ. 2012. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223. ( 10.1111/j.2041-210X.2011.00169.x) [DOI] [Google Scholar]
- 41.Hijmans RJ, van Etten J. 2014. raster: Geographic data analysis and modelling. R package, 2.2-12 ed. See https://cran.r-project.org/web/packages/raster/index.html. [Google Scholar]
- 42.Dray S, Dufour A-B. 2007. The ade4 package: implementing the duality diagram for ecologists. J. Stat. Softw. 22, 1–20. ( 10.18637/jss.v022.i04) [DOI] [Google Scholar]
- 43.Peres-Neto PR, Jackson DA. 2001. How well do multivariate data sets match? The advantages of a Procrustean superimposition approach over the Mantel test. Oecologia 129, 169–178. ( 10.1007/s004420100720) [DOI] [PubMed] [Google Scholar]
- 44.Oksanen J, et al. 2013. vegan: Community ecology package. R package version 2.0-10. See http://CRAN.R-project.org/package=vegan .
- 45.Günduz İ, Auffray J-C, Britton-Davidian J, Catalan J, Ganem G, Ramalhinho MG, Mathias ML, Searle JB. 2001. Molecular studies on the colonization of the Madeiran archipelago by house mice. Mol. Ecol. 10, 2023–2029. ( 10.1046/j.0962-1083.2001.01346.x) [DOI] [PubMed] [Google Scholar]
- 46.Macholan M. 2006. A geometric morphometric analysis of the shape of the first upper molar in mice of the genus Mus (Muridae, Rodentia). J. Zool. 270, 672–681. ( 10.1111/j.1469-7998.2006.00156.x) [DOI] [Google Scholar]
- 47.Misonne X. 1969. African and Indo-Australian Muridae. Evolutionary trends; 219 p. Tervuren, Belgique: Musée Royal de l'Afrique Centrale. [Google Scholar]
- 48.Renaud S, Pantalacci S, Quéré J-P, Laudet V, Auffray J-C. 2009. Developmental constraints revealed by co-variation within and among molar rows in two murine rodents. Evol. Dev. 11, 590–602. ( 10.111/j.1525-142X.2009.00365.x) [DOI] [PubMed] [Google Scholar]
- 49.Granjon L, Cheylan G. 1988. Mécanismes de coexistence dans une guilde de muridés insulaire (Rattus rattus L, Apodemus sylvaticus L. et Mus musculus domesticus Rutty) en Corse: conséquences évolutives. Z. Säugetierkunde 53, 301–316. [Google Scholar]
- 50.Nunes AC, Britton-Davidian J, Catalan J, Ramalhinho MG, Capela R, Mathias ML, Ganem G. 2005. Influence of physical environmental characteristics and anthropogenic factors on the position and structure of a contact zone between two chromosomal races of the house mouse on the island of Madeira (North Atlantic, Portugal). J. Biogeography 32, 2123–2134. ( 10.1111/j.1365-2699.2005.01337.x) [DOI] [Google Scholar]
- 51.Gómez Cano AR, Fernández MH, Álvarez-Sierra MÁ. 2013. Dietary ecology of Murinae (Muridae, Rodentia): a geometric morphometric approach. PLoS ONE 8, e79080 ( 10.1371/journal.pone.0079080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Le Roux V, Chapuis J-L, Frenot Y, Vernon P. 2002. Diet of the house mouse (Mus musculus) on Guillou Island, Kerguelen archipelago, Subantarctic. Polar Biol. 25, 49–57. ( 10.1007/s003000100310) [DOI] [Google Scholar]
- 53.Smith VR, Avenant NL, Chown SL. 2002. The diet and impact of house mice on a sub-Antarctic island. Polar Biol. 25, 703–715. ( 10.1007/s00300-002-0405-8) [DOI] [Google Scholar]
- 54.van Aarde RJ, Jackson TP. 2007. Food, reproduction and survival in mice on sub-Antarctic Marion Island. Polar Biol. 30, 503–511. ( 10.1007/s00300-006-0209-3) [DOI] [Google Scholar]
- 55.Ganem G. 2012. Behavior, ecology, and speciation in the house mouse. In Evolution of the house mouse (eds Macholan M, Baird SJE, Munclinger P, Pialek J), pp. 373–406. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 56.Chapuis J-L, Boussès P, Barnaud G.. 1994. Alien mammals, impact and management in the French subantarctic islands. Biol. Conserv. 67, 97–104. ( 10.1016/0006-3207(94)90353-0) [DOI] [Google Scholar]
- 57.Reisinger RR, De Bruyn PJN, Tosh CA, Oosthuizen WC, Mufanadzo NT, Bester MN. 2011. Prey and seasonal abundance of killer whales at sub-Antarctic Marion Island. Afr. J. Mar. Sci. 33, 99–105. ( 10.2989/1814232X.2011.572356) [DOI] [Google Scholar]
- 58.Millien V. 2006. Morphological evolution is accelerated among island mammals. PLoS Biol. 4, e321 ( 10.1371/journal.pbio.0040321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cucchi T, et al. 2014. The changing pace of insular life: 5000 years of microevolution in the Orkney vole (Microtus arvalis orcadensis). Evolution 68, 2804–2820. ( 10.1111/evo.12476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caumul R, Polly PD. 2005. Phylogenetic and environmental components of morphological variation: skull, mandible, and molar shape in marmots (Marmota, Rodentia). Evolution 59, 2460–2472. ( 10.1111/j.0014-3820.2005.tb00955.x) [DOI] [PubMed] [Google Scholar]
- 61.Renaud S, Auffray J-C.. 2013. The direction of main phenotypic variance as a channel to morphological evolution: case studies in murine rodents. Hystrix Ital. J. Mammal. 24, 85–93. ( 10.4404/hystrix-24.1-6296) [DOI] [Google Scholar]
- 62.Schluter D. 1996. Adaptive radiation along genetic lines of least resistance. Evolution 50, 1766–1774. ( 10.2307/2410734) [DOI] [PubMed] [Google Scholar]
- 63.Polly PD. 2001. On morphological clocks and paleophylogeography: towards a timescale for Sorex hybrid zones. Genetica 112–113, 339–357. ( 10.1023/A:1013395907225) [DOI] [PubMed] [Google Scholar]
- 64.Salazar-Ciudad I, Marin-Riera M.. 2013. Adaptive dynamics under development-based genotype–phenotype maps. Nature 497, 361–364. ( 10.1038/nature12142) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new sequences were deposited, as the newly generated sequences all corresponded to sequences already available in GenBank. The dataset including morphometric, climatic, ecologic and genetic data is available as the electronic supplementary material.