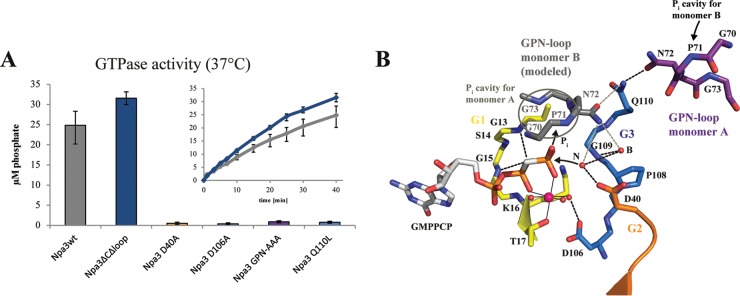
FIG 4.
Catalytic mechanism. (A) GTPase activity of Npa3 mutants. Bars represent free orthophosphate concentrations after 40 min at 37°C. Kinetics are shown for wild-type Npa3 (Npa3wt) (gray) and the crystallized variant Npa3ΔCΔLoop (blue). (B) Schematic of the mechanism of GTP hydrolysis. The active site of Npa3-GMPPCP is shown. The GPN loop of monomer B is modeled by superpositioning of two Npa3-GMPPCP enzymes on the archaeal Pab0955 dimer (PDB accession number 1YRB) (18). The nucleophilic water (N) that attacks the γ-phosphate and the buttressing water (B) are superimposed from the Npa3-GDP structure. Hydrogen bonds are shown as dashed black lines, potential hydrogen bonds derived from dimer modeling are shown as dashed gray lines, water molecules are shown as red spheres, the magnesium ion is shown as a pink sphere, and metal ion-ligand interactions are shown as solid black lines.