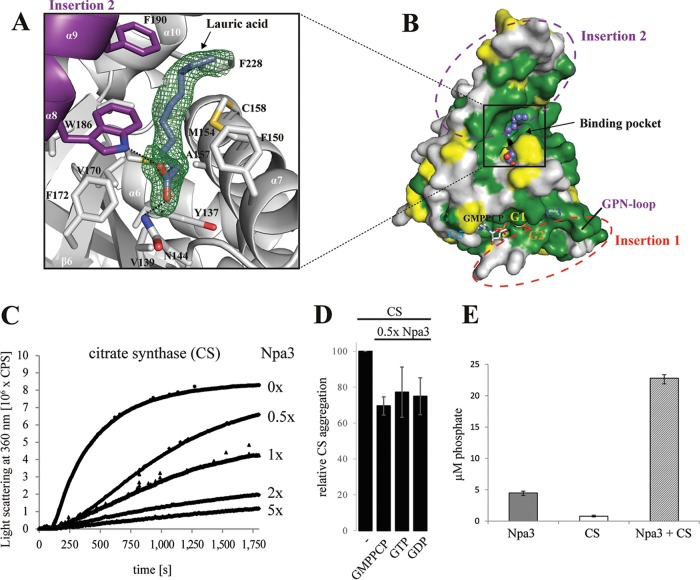
FIG 5.
Putative peptide-binding pocket and GTPase-stimulating chaperone activity. (A) Initial unbiased Fo-Fc difference electron density for lauric acid (slate blue sticks) bound to the putative peptide-binding pocket of Npa3, contoured at 2σ (green mesh). (B) Highly conserved surface of the putative peptide-binding pocket. Invariant residues are in green, conserved residues are in yellow, and variable residues are in gray. Insertion regions and G motifs are depicted. Lauric acid is shown as blue spheres. (C) Npa3 has chaperone-like activity in vitro. Npa3 suppresses thermally induced (43°C) aggregation of the general nonnative chaperone substrate protein citrate synthase (CS). Different amounts of wild-type Npa3 were added, as indicated on the right. (D) Chaperone-like activity of Npa3 is independent of added nucleotides under limiting conditions. A total of 1 mM GMPPCP, GTP, or GDP was added, and relative CS aggregation in the presence of 0.5× molar amounts of Npa3 was determined after 30 min. (E) GTPase activity of Npa3 is stimulated >4-fold in the presence of the nonnative chaperone-substrate protein citrate synthase. Free orthophosphate concentrations were determined after 40 min at 43°C (see Materials and Methods).