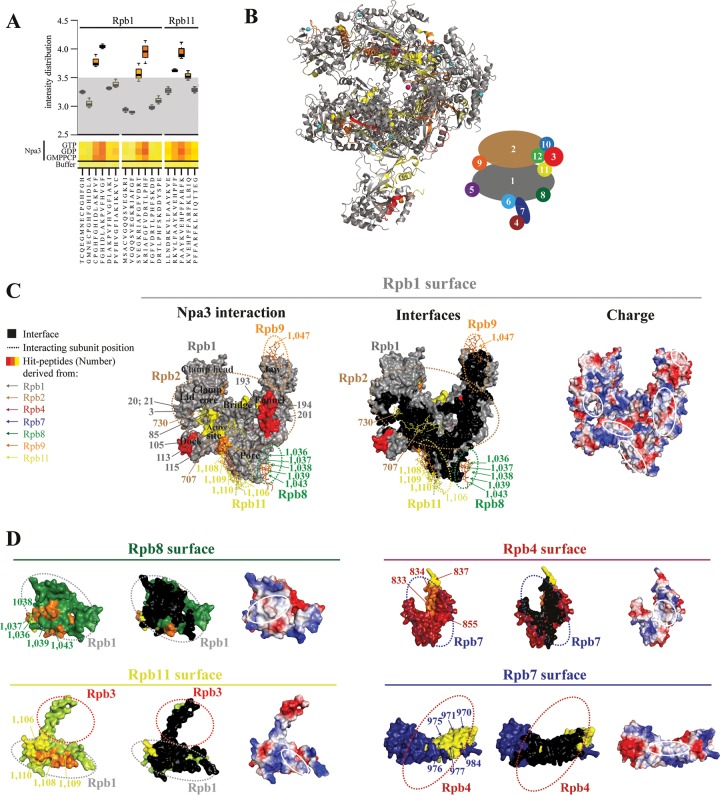
FIG 6.
Npa3 binds Pol II-derived peptides located at subunit interfaces. (A) Box plot representation of a representative portion of the heat map describing the Npa3 peptide-binding landscape. Tested were a total of 1,139 15-mer Pol II-derived peptides, covering the complete sequence of the 12-subunit Pol II in the presence of GMPPCP, GTP, and GDP. Control experiments were performed without Npa3 and nucleotides to test the cross-reactivity of the anti-His antibody. Intensity distribution is shown on a logarithmic scale. Peptides with a signal intensity of <3.5 were defined as unbound peptides (gray area). (B) Location of Npa3-binding peptides in the assembled Pol II complex (PDB accession number 1WCM) (32). Npa3 binding to Pol II peptides is depicted in yellow (signal intensity of 3.5 to 3.75), orange (3.75 to 4), and red (>4), whereas unbound regions are in gray (<3.5). A schematic representation of the 12 Pol II subunits Rpb1 to Rpb12 in the folded Pol II complex is shown on the right. (C and D) Surface representations of Pol II subunits showing that Npa3 interacts with hydrophobic Pol II-derived peptides located at subunit interfaces (left). Peptides in Pol II subunit surfaces involved in interactions with other subunits are colored as described above for panel B. Numbers correspond to the peptide numbers from the array (see Table S1 in the supplemental material for a list of all peptides, Table S2 in the supplemental material for a list of all peptides that interact with Npa3, and Fig. S4 in the supplemental material for original peptide interaction analysis) and are color-coded according to the subunit from which they are derived, as shown on the left and in the schematic of Pol II subunit organization in panel B. Npa3-bound peptides from other subunits that interact with Rpb1 are shown as sticks. Residues involved in subunit interfaces are shown in black (middle). White solid lines highlight hydrophobic interface regions, bound by Npa3 (right). Dashed lines indicate where other Pol II subunits are positioned in the assembled complex.