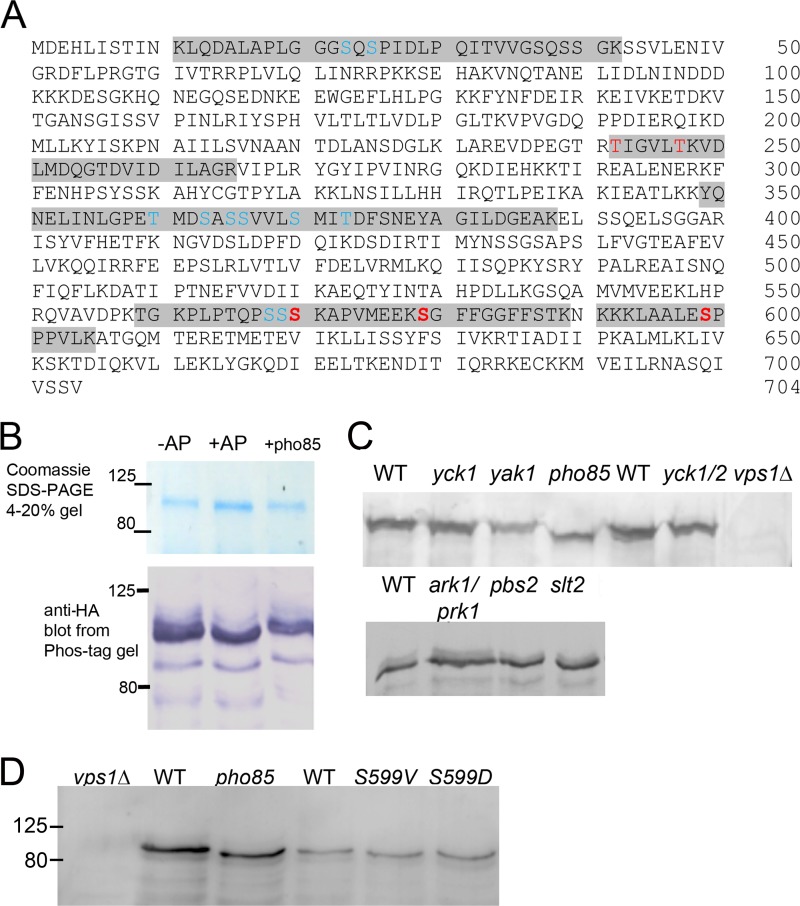
FIG 1.
Vps1 phosphorylation in vivo and in vitro. (A) Vps1-HA was immunoprecipitated from log-phase cells and subjected to analysis by mass spectrometry as described in the text. Peptides carrying phosphorylated residues are highlighted. High-confidence sites are shown in red (high-confidence identity, PEP < 0.01; high-confidence localization, 1% false-localization rate). Other sites (blue) were identified either with medium confidence (PEP > 0.01) but with high-confidence localization or with high confidence identity (PEP < 0.01) but low-confidence localization (>1% false-localization rate). (B) Immunoprecipitated Vps1 was incubated in the presence (+) or absence (−) of calf intestinal AP. Following AP treatment, a fraction of the HA-tagged Vps1 was incubated in the presence of GST-Pho85, as described in the text. Extracts were separated both on an Any Kd gradient gel or on SDS-7.5% PAGE containing 20 μM Phos-tag additive, followed by blotting and probing for Vps1 with anti-HA antibodies. (C) Whole-cell extracts were made from strains with deletions of kinase genes. Extracts were separated on gels (10% gel; 100 μM Phos-tag) and blotted as described in the text. The blots were probed with anti-Vps1 antibodies. WT, wild type. (D) The Pho85 consensus site at Ser599 was mutagenized to valine or aspartate. Extracts from cells expressing these were made, separated, blotted, and probed with antibody. The pho85 deletion and vps1 mutants were not isogenic, so different wild-type strains were used for extracts. Numbers indicate the sizes of molecular weight markers.