Abstract
Tissue fibrosis is a major cause of organ dysfunction during chronic diseases and aging. A critical step in this process is transforming growth factor β1 (TGF-β1)-mediated transformation of fibroblasts into myofibroblasts, cells capable of synthesizing extracellular matrix. Here, we show that SIRT3 controls transformation of fibroblasts into myofibroblasts via suppressing the profibrotic TGF-β1 signaling. We found that Sirt3 knockout (KO) mice with age develop tissue fibrosis of multiple organs, including heart, liver, kidney, and lungs but not whole-body SIRT3-overexpressing mice. SIRT3 deficiency caused induction of TGF-β1 expression and hyperacetylation of glycogen synthase kinase 3β (GSK3β) at residue K15, which negatively regulated GSK3β activity to phosphorylate the substrates Smad3 and β-catenin. Reduced phosphorylation led to stabilization and activation of these transcription factors regulating expression of the profibrotic genes. SIRT3 deacetylated and activated GSK3β and thereby blocked TGF-β1 signaling and tissue fibrosis. These data reveal a new role of SIRT3 to negatively regulate aging-associated tissue fibrosis and discloses a novel phosphorylation-independent mechanism controlling the catalytic activity of GSK3β.
INTRODUCTION
Fibrosis refers to the formation of excess fibrous tissue, which contributes to morbidity and mortality associated with organ failure in response to chronic diseases and/or injury. Tissue fibrosis is also a hallmark of the aging process. In the developed world, fibrotic diseases account for nearly 45% of human deaths, yet there are no approved therapies to date which can arrest or prevent tissue fibrosis (1).
At the molecular level, fibrosis occurs because of the development of myofibroblasts, activated fibroblast-like cells capable of synthesizing exaggerated amounts of extracellular matrix (ECM) and contractile proteins (2). These activated myofibroblasts also synthesize and secrete growth factors, cytokines, and inflammatory mediators to promote fibrosis. Although myofibroblasts can arise from many different cell types, their most common source is local quiescent tissue fibroblasts, which are activated in response to stress stimuli and/or injury. During the normal wound-healing process, myofibroblasts undergo apoptosis after wound repair, leaving a healed scar with low cellular content. But in response to chronic diseases and tissue aging, a multifold increase of myofibroblasts occurs, and they are constantly generated without undergoing apoptosis. Excessive generation of myofibroblasts causes persistent deposition of fibrous tissue leading to organ failure (3). The molecular signals which induce persistent generation of myofibroblasts and which make these cells resistant to apoptosis are not yet understood.
One of the major contributors of tissue fibrosis is activation of transforming growth factor β (TGF-β) signaling (2). The TGF-β superfamily consists of three ligands, TGF-β1, -β2, and -β3, which are synthesized as latent precursors. Activated TGF-β binds to membrane receptors and initiates a series of phosphorylation-dependent signaling cascades which finally culminate in activation of the Smad family of transcription factors (1). These factors in combination with other accessory factors regulate the expression of genes which lead to transformation of fibroblasts into myofibroblasts and induction of fibrosis. During aging several components of TGF-β signaling are amplified which facilitate the process of tissue fibrosis. Similarly, during chronic stress and/or injury, sustained synthesis of TGF-β1 has been reported in many tissues (1). However, molecular signals which determine prolonged synthesis of TGF-β1 are not yet completely understood.
One of the signaling kinases which interferes with TGF-β-signaling is glycogen synthase kinase 3β (GSK3β). GSK3 is a serine/threonine kinase which regulates a wide variety of cellular functions (4). GSK3 is expressed in two isoforms, GSK3α and GSK3β. Both are highly conserved, ubiquitously expressed, and possess unique as well as overlapping functions. While GSK3β has been shown to be localized in the cytoplasm, in the nucleus, and in mitochondria, GSK3α has not been reported in mitochondria (5). Unlike other kinases, GSK3β is active in the resting state and negatively regulates cellular growth. Upon growth factor stimulation of cells, GSK3β is phosphorylated at N-terminal serine (S9) residues by the upstream kinases, such as Akt, leading to inhibition of GSK3β activity and thereby removing its negative control of cellular growth. However, data obtained from GSK3β-S9A knock-in mice have indicated that these mice are not resistant to GSK3β inhibition in response to growth stimuli. This suggested that GSK3β might also be inhibited by mechanisms independent of serine phosphorylation (6). Besides phosphorylation, no other posttranslational modification has been demonstrated so far which could explain inhibition of GSK3β activity during growth factor stimulation of cells.
Sirtuins are class III histone deacetylases (HDACs) which need NAD for their deacetylation reactions. There are seven sirtuin isoforms (SIRT1 to SIRT7) expressed in mammalian cells. These isoforms are localized in different subcellular compartments (7). Among them, SIRT3 is primarily localized in mitochondria, and its levels are elevated by calorie restriction and endurance exercise (8). SIRT3 activation has been shown to protect mice from developing heart failure, cancer, metabolic syndrome, and aging-associated hearing loss (9–12). SIRT3 is also reported as an essential regulator of hematopoietic stem cell aging (13). In another study, polymorphism in the coding sequences causing reduced SIRT3 activity was linked to increased susceptibility to develop obesity and diabetes, thus suggesting a role of SIRT3 in the human aging process (10).
In this study, we demonstrate that SIRT3 negatively regulates tissue fibrosis via activating GSK3β and blocking synthesis of TGF-β1. SIRT3 deficiency induces hyperacetylation of GSK3β, resulting in impaired activity of GSK3β to phosphorylate its substrates. Reduced enzymatic activity of GSK3β leads to stabilization and activation of transcription factors involved in the process of tissue fibrosis.
MATERIALS AND METHODS
Antibodies, plasmids, and viral vectors.
Antibodies detecting collagen 1, acetylated lysine (Ac-lysine), phospho-CREB (p-CREB), and manganese-containing superoxide dismutase (MnSOD) were purchased from Millipore, Inc. Specific antibodies against TGF-β1 and Flag tag were purchased from Abcam. Antibodies to tubulin, actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), fibronectin, SIRT3, c-Jun, Ac-lysine, Smad2/3, p65, NFATc, p-GSK3-β T216, axin, p300, c-Myc, and β-catenin were purchased from Santa Cruz. Antibodies to smooth muscle actin (SMA), vimentin, SIRT5, c-Myc, and Flag-agarose beads were obtained from Sigma-Aldrich. Antibodies to SIRT3, GSK3β, p-GSK3β, c-Jun, Smad3, p-Smad3, Ac-lysine, p-β-catenin, glycogen synthase, and p-glycogen synthase were purchased from Cell Signaling. GSK3α/β-specific antibody was obtained from Epitomics. Phospho-Smad3 T66 antibody was a kind gift from Xiao-Fan Wang, Duke University Medical Center, Durham, NC. A hemagglutinin (HA)-GSK3β construct was obtained from Addgene, and a series of point mutations were performed in this construct with a QuikChange Lightning site-directed mutagenesis kit (Agilent Technologies). Recombinant p300/CBP-associated factor (PCAF) and p300 proteins were purchased from Millipore. A TGF-β/activin response element-luciferase (TARE-Luc) reporter plasmid was bought from Stratagene, Inc. Flag-tagged SIRT3 (Flag-SIRT3) and Flag-SIRT3 catalytic mutant constructs were described previously (14). Adenoviruses for recombinant human SIRT3 and SIRT5 were purchased from Vector Biolabs. Adenovirus vectors expressing a short hairpin RNA (shRNA) against p300 was kindly provided by B. Thimmapaya (Northwestern University, Chicago, IL).
Human heart samples.
All procedures involving human heart samples were performed in compliance with institutional guidelines for human research and were approved by the Institutional Review Board of the University of Chicago. Human heart samples were obtained from the cardiac transplant program of the University of Chicago. Failing heart specimens were obtained from diseased hearts that were removed during orthotopic heart transplantation. The detailed protocols for the isolation and culture of failing cardiac fibroblasts were described previously (15). Adult human nonfailing left ventricular control cardiac fibroblasts were purchased from Cell Applications, Inc. (San Diego, CA). Six different human fibroblast cultures were obtained as controls. Cultured fibroblasts were used within a few passages of culturing to ensure preservation of the control or failing phenotype (15).
Animal experiments.
All animal protocols were reviewed and approved by the University of Chicago Institutional Animal Care and Use Committee. Mice with cardiac tissue-restricted expression of Sirt3 and the infusion of Ang-II in mice to develop hypertrophy and fibrosis were described previously (12). Cardiac fibroblasts from 6-month-old wild-type (WT) and Sirt3-knockout (KO) mice were isolated and cultured as previously described (15). SB-505124 (S4696; Sigma) was dissolved in dimethyl sulfoxide (DMSO), which was further diluted with peanut oil and administered at a dose of 10 mg per kg body weight, intraperitoneally (i.p.), three times a week. The hearts of WT and Sirt3-KO mice were analyzed for fibrosis after 2 months of treatment with vehicle or SB-505124 by Masson trichrome staining as described previously (16).
Generation of the wSirt3-Tg mice.
Whole-body Sirt3-transgenic (wSirt3-Tg) mice were generated by crossing loxP-stop-LoxP-SIRT3.Flag-transgenic mice with mice expressing Cre under the control of the human β-actin promoter as described elsewhere (17).
General methods.
A GSK3-β activity assay was performed using a commercially available GSK3β activity assay kit (CS0990-1KT; Sigma). Protocols for immunostaining, confocal microscopy, Western blotting, trichrome staining of tissue sections, real-time PCR, luciferase reporter assays, coimmunoprecipitation, chromatin immunoprecipitation (ChIP), in vitro deacetylation assays, in vitro protein-binding assays, site-directed mutagenesis, mass spectroscopy, flow cytometry-based reactive oxygen species (ROS) measurement, and subcellular fractionation are described in our previous publications (12, 16, 18–20).
In vitro assay of SIRT3 activity.
To assay for SIRT3 enzymatic activity, we used a slight modification of a previously published protocol (10). Briefly, SIRT3 was immunoprecipitated using anti-FLAG M2-agarose antibody and mouse liver extracts. One hundred nanograms of immunoprecipitated SIRT3 was added to a 100-μl reaction mixture containing 50 mM Tris, pH 8.5, 4 mM MgCl2, 0.2 mM dithiothreitol (DTT), and 50 mM NAD+. 3H-labeled acetylated histone H4 peptide (5,000 cpm) was added to the mixture and incubated for 30 min. The reaction was stopped with an equal volume of 2× stop solution (0.2 M HCl and 0.32 M acetic acid). The mixture was mixed with 500 μl of ethyl acetate and centrifuged at 14,000 × g for 5 min. A total of 450 μl of the upper-phase solution was transferred to 5 ml of scintillation solution, and counts were determined using a Beckman LS6000SC instrument.
Statistical analysis.
Statistical differences among groups were determined using either Student's t test (for two groups) or one-way analysis of variance (ANOVA) (for more than two groups) by use of GraphPad Prism software.
RESULTS
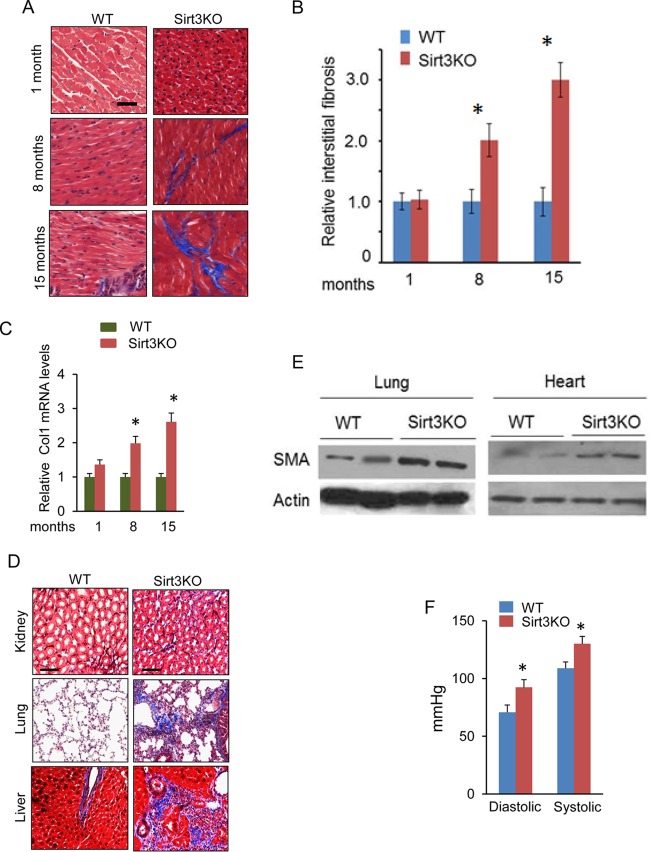
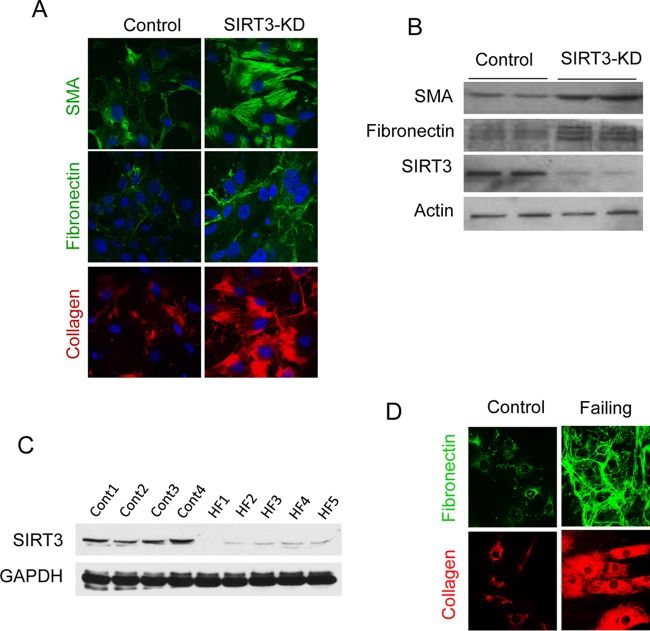
We along with others have previously reported that although Sirt3-deficient (Sirt3-KO) mice look normal at birth, they develop cardiac hypertrophy and contractile dysfunction in adulthood (12). To determine the consequences of SIRT3 deficiency on aging of the heart, we analyzed cardiac fibrosis in three age groups (1, 8, and 15 months old) of Sirt3-KO mice. The results showed that there was increased cardiac fibrosis with aging of Sirt3-KO mice (129sv) (Fig. 1A to C). We also analyzed tissue fibrosis in other organ systems and found that, with age, Sirt3-KO mice developed tissue fibrosis in multiple organs, including liver, lung, and kidney, in contrast to their age-matched wild-type (WT) controls (Fig. 1D and E). We also measured blood pressure (BP) of these mice. Sirt3-KO mice had ∼25-mm Hg higher arterial blood pressure than the controls (Fig. 1F). To test whether Sirt3 deficiency directly induces fibrosis or whether it is secondary to increased BP, we depleted Sirt3 from control human cardiac fibroblasts using a small interfering RNA (siRNA) and analyzed the expression of myofibroblast markers. There was increased expression of the myofibroblast markers collagen 1 (Col1), fibronectin, and smooth muscle α-actin (SMA) in Sirt3-depleted fibroblasts (Fig. 2A and B). We also analyzed SIRT3 levels in fibroblasts isolated from hearts of patients with end-stage heart failure. Fibroblasts of failing hearts showed reduced SIRT3 levels and robust expression of the myofibroblast markers collagen 1 and fibronectin compared to levels in controls (Fig. 2C and D). These findings indicated that SIRT3 deficiency contributes to development of age-dependent organ fibrosis due to sensitization of fibroblasts to transform into myofibroblasts.
FIG 1.
Reduced SIRT3 levels contribute to tissue fibrosis. (A) Comparative study of fibrosis in hearts, stained with Masson's trichrome stain (blue), of Sirt3-KO mice of different age groups and their age-matched wild-type (WT) controls. (B and C) Quantification of cardiac fibrosis and Col1 mRNA levels in Sirt3-KO and WT mice of different age groups. Values are means ± standard errors (n = 4). *, P < 0.01. (D) Tissue sections of 15-month-old WT and Sirt3-KO mice, stained to detect fibrosis (sections are representative of 5 mice per group). (E) Expression of the fibrotic marker SMA in the lung and heart tissue samples collected from 15-month-old Sirt3-KO and WT mice. (F) Systemic arterial pressure (femoral artery) in 15-months-old WT and Sirt3-KO mice. Values are means ± standard errors (n = 10). *, P < 0.001.
FIG 2.
SIRT3 deficiency promotes transformation of human cardiac fibroblasts into myofibroblasts. (A and B) Expression of myofibroblast markers in control and SIRT3-depleted (SIRT3-KD) human cardiac fibroblasts as analyzed by confocal microscopy and Western blotting. (C) Western blot analysis showing SIRT3 levels in human cardiac fibroblasts isolated from controls and patients with heart failure (HF). (D) Confocal microscopy showing expression of myofibroblast markers in human cardiac fibroblasts isolated from control and failing hearts.
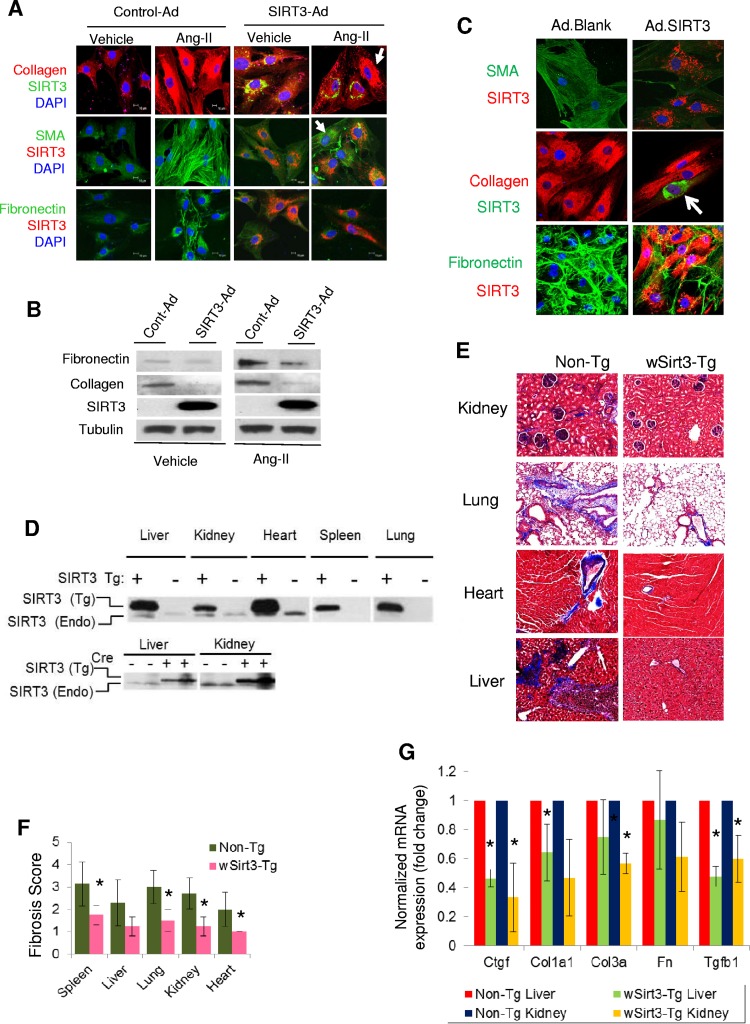
We next studied the effect of SIRT3 overexpression in mouse cardiac fibroblasts stimulated with angiotensin II (Ang-II), a profibrotic agent. Treatment of fibroblasts with Ang-II markedly increased expression of myofibroblast markers in control cells but not in SIRT3-overexpressing cells (Fig. 3A and B). We also found that SIRT3 overexpression blocked the expression of fibrotic markers (SMA, collagen, and fibronectin) in myofibroblasts prepared from failing human hearts (Fig. 3C). To explore the antifibrotic role of Sirt3 in an in vivo model, we analyzed tissue fibrosis in Sirt3-transgenic (Sirt3-Tg) mice having whole-body Sirt3 overexpression (∼4-fold) (Fig. 3D). Different tissue samples from 15-month-old nontransgenic and Sirt3-Tg mice were collected at the University of California, San Francisco (UCSF), and shipped without identifiers (codes) to the University of Chicago. This blinded analysis revealed that Sirt3-Tg mice have reduced fibrosis in multiple organs, including the heart, liver, kidney, lung, and spleen, compared to levels in their age-matched controls (Fig. 3E and F). These Sirt3-Tg mice also showed significantly reduced expression levels of several fibrotic markers, including collagen 1, collagen 3, connective tissue growth factor (CTGF), and TGF-β1 (Fig. 3G). These results are consistent with our previous findings where Sirt3-transgenic hearts were found resistant to developing Ang-II-mediated cardiac fibrosis (12). Together, these results indicated that Sirt3 activation prevents development of aging-associated tissue fibrosis.
FIG 3.
SIRT3 overexpression blocks aging-associated tissue fibrosis. (A) Mouse cardiac fibroblasts were overexpressed with control or adenovirus (Ad) vector synthesizing SIRT3. The next day, cells were stimulated with Ang-II (200 nM) for 48 h. The expression of myofibroblast markers was analyzed by confocal microscopy. Note the reduced expression of collagen, SMA, and fibronectin in SIRT3-overexpressing cells but not in cells lacking SIRT3 overexpression (arrows). DAPI, 4′,6′-diamidino-2-phenylindole. (B) Western blot analysis showing expression of the indicated proteins in mouse cardiac fibroblasts subjected to SIRT3 overexpression and stimulated with Ang-II. All four group samples were run in the same gel. (C) Cardiac myofibroblasts isolated from patients with heart failure were infected with control (blank) or SIRT3 adenovirus. The expression of myofibroblast markers (SMA, collagen, and fibronectin) was analyzed by confocal microscopy. SIRT3 expression was verified by immunostaining for SIRT3 (red) in SMA and fibronectin panels and in collagen panels (green). Note that SIRT3 overexpression (arrow) blocked the expression of collagen. (D) The Cre-recombinase-dependent overexpression of SIRT3-FLAG in different organs was detected by Western blotting using a specific antiserum for mouse SIRT3. The endogenous (Endo) SIRT3 protein (lacking a tag) is present as a lower-abundance and lower-molecular-weight protein. (E and F) Tissue fibrosis was analyzed in 15-month-old nontransgenic (Non-Tg) and whole-body Sirt3-transgenic (wSirt3-Tg) mice (C57BL/6). Tissue sections were stained with Masson's trichrome stain (E), and relative fibrosis was scored (F) in a blinded fashion (n = 4 to 6 mice per group). (G) Real-time PCR analysis of mRNA of fibrosis-related genes in the liver and kidney samples of non-Tg and wSirt3-Tg mice. Values are means ± standard errors (n = 4 to 7 mice per group). *, P < 0.001.
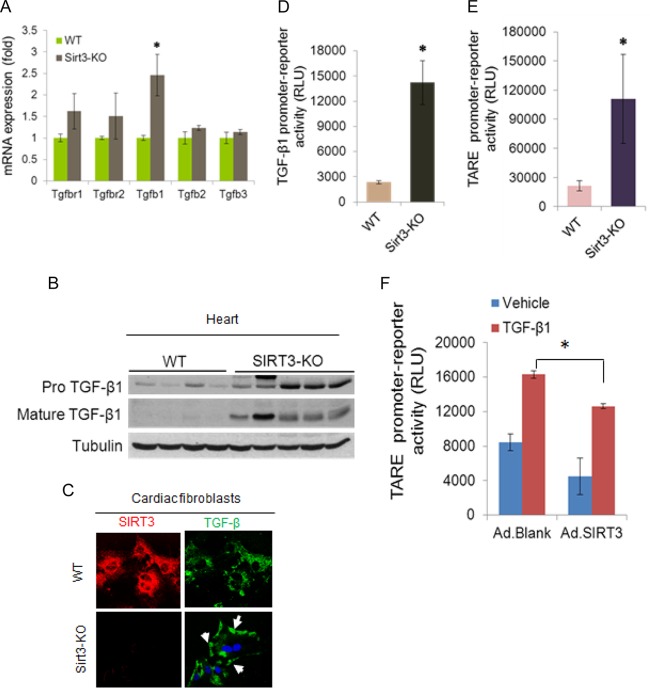
A major contributor of tissue fibrosis is activation of TGF-β1 signaling, which is amplified during aging (21). There are also reports showing that TGF-β signaling could be suppressed by calorie restriction (CR) (22). Since SIRT3 is capable of blocking tissue fibrosis and since it is activated by CR, we posited that it might regulate TGF-β signaling. Real-time PCR analysis showed increased TGF-β1 mRNA expression in Sirt3-KO hearts of 8-month-old mice (Fig. 4A), as well as in isolated cardiac fibroblasts (data not shown). Sirt3-KO hearts also showed increased expression of both unprocessed and mature forms of TGF-β1 (Fig. 4B), and it was predominantly localized in the cell membrane of Sirt3-KO fibroblasts, a characteristic of TGF-β1 signaling (Fig. 4C). Sirt3-KO cardiac fibroblasts also exhibited increased transcriptional activity of the natural TGF-β1 promoter (Fig. 4D). Consistent with these findings, Sirt3-KO cells exhibited increased activity of a promoter/reporter gene having multiple TGF-β1-responsive elements (TARE) (Fig. 4E). The TGF-β1-induced activity of this promoter/reporter gene was blocked by overexpression of SIRT3 (Fig. 4F), thus demonstrating a role of Sirt3 in regulating TGF-β1 synthesis.
FIG 4.
SIRT3 deficiency activates TGF-β1 synthesis. (A) Reverse transcription-PCR analysis showing expression of different members of the TGF-β family ligands and receptors (r) in the hearts of adult (8 months old) WT and Sirt3-KO mice. Values are means ± standard errors (n = 5 mice per group). *, P < 0.001. (B) Western blot analysis of pro-TGF-β1 and mature TGF-β1 in mouse hearts (n = 4 or 5 mice per group). (C) Cells were immunostained for SIRT3 and TGF-β1 and imaged by confocal microscopy. Note the increased amount of membrane-bound TGF-β1 (arrows) in Sirt3-KO fibroblasts. (D) A luciferase reporter assay showing activity of the TGF-β1 natural promoter in cardiac fibroblast of WT and Sirt3-KO hearts. Values are means ± standard errors (n = 3). *, P < 0.001). (E) A luciferase reporter assay was performed with a synthetic promoter containing multiple TGF-β/activin response elements (TAREs) in cardiac fibroblasts of WT and Sirt3-KO mice. Values are means ± standard errors (n = 6). *, P < 0.001). (F) The TARE promoter/reporter plasmid was transfected into control and SIRT3-overexpressing cardiac fibroblasts. Cells were stimulated with vehicle or 10 nM recombinant TGF-β1 for 12 h, and the luciferase activity was measured. Values are means ± standard errors (n = 3). *, P < 0.001. RLU, relative light units.
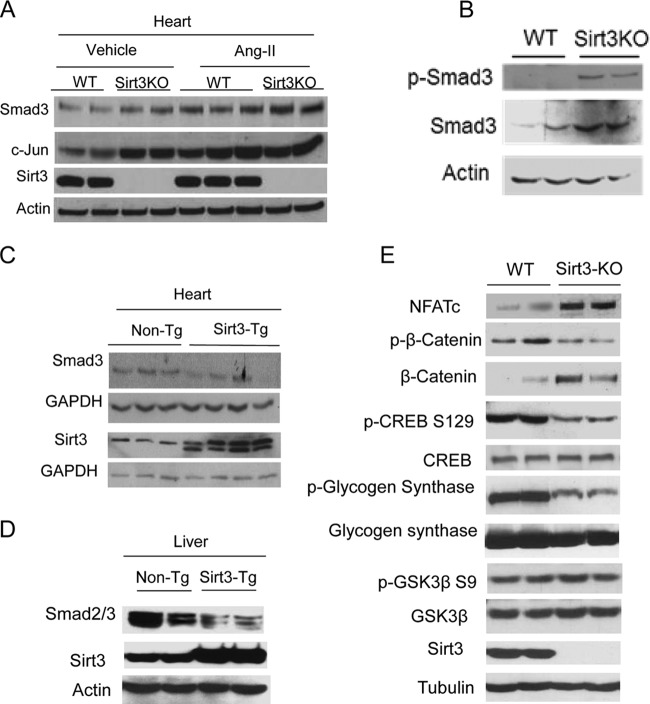
To determine the underlying mechanism which regulates TGF-β1 signaling in Sirt3-KO cells, we analyzed expression levels of the transcription factors c-Jun and Smad3, both of which are linked to the TGF-β1 signaling pathway (synthesis and activity). As expected, Sirt3 deficiency led to increased levels of c-Jun and Smad3 in the mouse hearts, under both basal and Ang-II-stimulated conditions (Fig. 5A). Similarly, increased Smad3 levels were found in Sirt3-KO fibroblasts (Fig. 5B). We also measured Smad3 levels in the heart and liver of transgenic mice having Sirt3 overexpression. In both Sirt3-transgenic samples, Smad3 levels were significantly lower than those of nontransgenic controls (Fig. 5C and D). These results thus demonstrated that SIRT3 plays a role in regulating the TGF-β1 signaling.
FIG 5.
SIRT3 regulates expression of profibrotic transcription factors. (A) Western blot analysis of the indicated proteins in adult mouse hearts stimulated to develop cardiac hypertrophy and fibrosis by Ang-II infusion (n = 5 in each group). (B) Western blot analysis showing Smad3 levels in WT and Sirt3-KO cardiac fibroblasts. (C and D) Western blot analysis showing Smad3 levels in the heart and liver of nontransgenic (Non-Tg) and whole-body Sirt3-transgenic mice. Blots are representative of 5 animals in each group. (E) Western blot analysis showing expression levels of different GSK3β targets in WT and Sirt3-KO hearts (n = 5 mice in each group).
In addition to TGF-β signaling, tissue fibrosis can also be promoted by activation of transcription factors like β-catenin and NFATc (23–26). We therefore measured expression of these factors in Sirt3-KO hearts. We observed increased expression of β-catenin and NFATc in Sirt3-KO hearts compared to levels in WT controls (Fig. 5E). These results thus suggested that SIRT3 deficiency promotes expression of transcription factors, which are known to target fibrotic genes.
In a previous study, SIRT3 has been shown to bind to gene promoters and suppress their transcription (27). To test whether SIRT3 controls expression of fibrotic genes by binding to promoters, we analyzed SIRT3 binding to promoters of fibrotic genes. The results obtained from the ChIP analysis showed no binding of SIRT3 to promoters of TGF-β1 or other well-characterized fibrotic genes like SMA and Col1a (data not shown), thus excluding the possibility of SIRT3 regulating expression of fibrotic genes by binding to their promoters. Another possibility was increased ROS levels due to SIRT3 deficiency, which might contribute to activation of fibrotic genes. To test this possibility, we treated Sirt3-KO mouse cardiac fibroblasts having a myofibroblast phenotype with the antioxidant N-acetylcysteine, a general antioxidant, or with EUK-134, a SOD2 and catalase mimetic (28, 29). Neither antioxidant reduced expression levels of the myofibroblast markers SMA and fibronectin nor of Smad3 though each was capable of reducing the ROS levels of Sirt3-KO fibroblasts, thus indicating that ROS is not the primary cause of fibrosis during SIRT3 deficiency (data not shown).
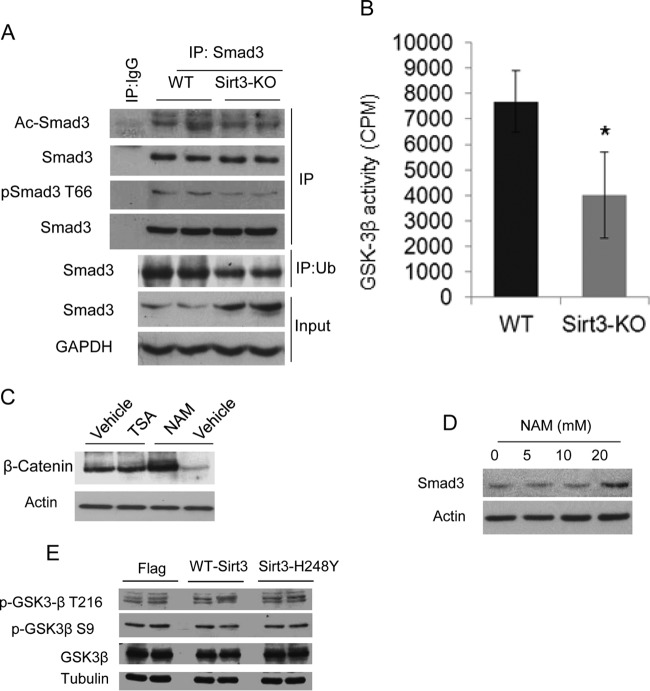
Smad3 is an acetylated protein, and the acetylation modification is known to promote transcriptional activity of Smad3 (30). We therefore asked whether SIRT3 deficiency enhances Smad3 acetylation due to cellular stress. However, we found no increase of Smad3 acetylation in Sirt3-KO hearts (Fig. 6A, upper panel). Smad3 is also known to be phosphorylated at threonine-66 (T66) by GSK3β, a modification which promotes Smad3 degradation by the ubiquitin-proteasome system. Decreased GSK3β-mediated phosphorylation stabilizes Smad3 and promotes its localization into the nucleus (31). Since we observed increased levels of Smad3 during SIRT3 deficiency (Fig. 5), we tested the phosphorylation and ubiquitination status of Smad3 in Sirt3-KO hearts. The results showed reduced T66 phosphorylation, which corresponded with reduced ubiquitination of Smad3 in Sirt3-KO hearts (Fig. 6A, lower panels). These experiments thus suggested reduced activity of GSK3β in Sirt3-KO cells.
FIG 6.
Sirt3 deficiency reduces GSK3β activity and stabilizes Smad3 levels. (A) Smad3 was immunoprecipitated (IP) from WT and Sirt3-KO hearts and analyzed for acetylation and T66 phosphorylation using specific antibodies. For this analysis, Smad3 levels were normalized. In the same lysate, ubiquitinated Smad3 was analyzed by immunoprecipitation with antiubiquitin (Ub) antibody, followed by Western blotting. (B) GSK3β prepared from heart lysates of WT and Sirt3-KO mice was tested for its ability to phosphorylate the glycogen synthase recombinant peptide. Values are mean ± standard errors (n = 7). *, P < 0.001. (C) Cardiac fibroblasts were treated with the HDAC inhibitor trichostatin A (TSA; 1 μM) or the pansirtuin inhibitor nicotinamide (NAM; 20 mM) overnight. The β-catenin levels were determined by Western blotting. (D) Western blot analysis showing Smad3 levels in fibroblasts treated with increasing concentrations of NAM. (E) Western analysis showing expression of phosphorylated GSK3β in cardiac fibroblasts subjected to overexpression of WT Sirt3 or an Sirt3 mutant.
To examine the role of SIRT3 in regulating GSK3β activity, we then measured the phosphorylation status of many targets of GSK3β. The results showed reduced phosphorylation of many well-established GSK3β targets, such as glycogen synthase, β-catenin, and CREB, in Sirt3-KO hearts (Fig. 5E), suggesting that Sirt3 deficiency curtails GSK3β activity. We also directly tested enzymatic activity of GSK3β prepared from Sirt3-KO hearts by using a recombinant peptide substrate. The results indicated reduced GSK3β catalytic activity in Sirt3-KO hearts compared to the level in controls (Fig. 6B). We then tested effect of a pan-sirtuin inhibitor, nicotinamide (NAM), on GSK3β targets. Treatment with NAM but not trichostatin A (TSA; a class I and II HDAC inhibitor) increased cellular levels of β-catenin and Smad3, thus again indicating a role of SIRT3 in regulating GSK3β activity (Fig. 6C and D). GSK3β activity is known to be reduced by N-terminal S9 phosphorylation, whereas it is activated by T216 phosphorylation. Our analysis showed that SIRT3 depletion or overexpression does not change phosphorylation of GSK3β at the S9 or T216 residue (Fig. 5E and 6E). These results thus demonstrated that SIRT3 regulates GSK3β activity in a phosphorylation-independent manner.
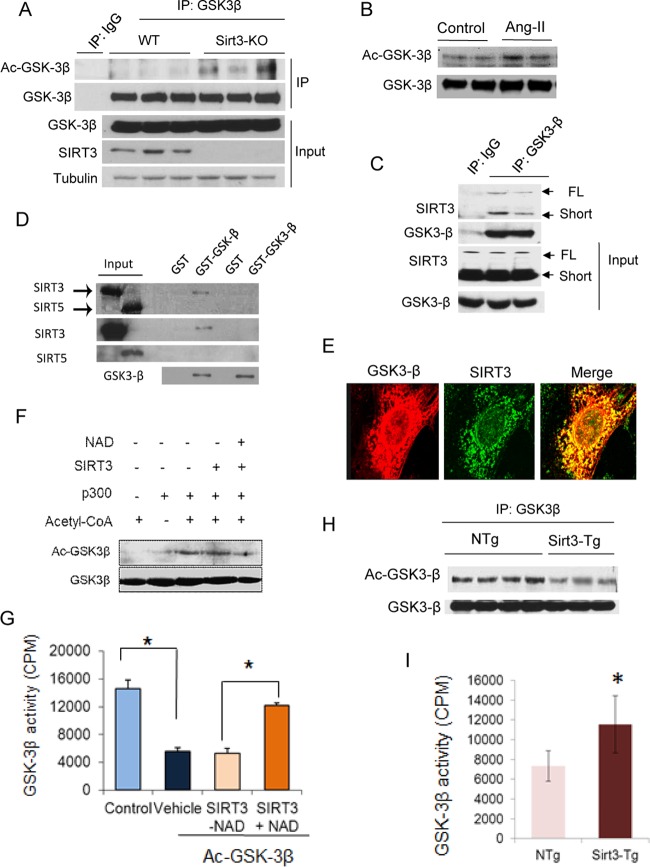
After determining that SIRT3 regulates GSK3β activity, we asked whether GSK3β could be modified by lysine acetylation. Immunoprecipitation studies revealed that GSK3β is acetylated in Sirt3-KO hearts (Fig. 7A). GSK3β was also acetylated in Ang-II-infused hearts that were stimulated to develop hypertrophy (Fig. 7B). Immunoprecipitation studies also showed that GSK3β was coprecipitated with both the full-length and short forms of SIRT3 (Fig. 7C). We also studied SIRT3 binding to GSK3β in a cell-free system. SIRT3, but not SIRT5 (a negative control), was pulled down by agarose beads containing glutathione S-transferase (GST)–GSK3β, thus indicating that SIRT3 directly binds to GSK3β (Fig. 7D). We next studied colocalization of these proteins. GSK3β was colocalized with SIRT3 in cardiac fibroblasts (Fig. 7E). Since SIRT3 is mostly present in mitochondria, these results also indicated that GSK3β and SIRT3 colocalized in mitochondria. Furthermore, in an in vitro assay, SIRT3 deacetylated and activated GSK3β in a NAD-dependent manner (Fig. 7F and G). Additionally, overexpression of SIRT3 deacetylated and activated GSK3β in transgenic mouse hearts (Fig. 7H and I). We also studied acetylation of GSK3β in Sirt1-KO hearts and found no change in protein acetylation (data not shown). These results together demonstrated that GSK3β is a new target of SIRT3.
FIG 7.
SIRT3 deacetylates and activates GSK3β. (A and B) GSK3β was immunoprecipitated from heart lysates of WT and Sirt3-KO mice (A) or mice stimulated to develop hypertrophy by Ang-II infusion (B). The precipitate was analyzed by Western blot analysis using antiacetyllysine antibody. (C) Coimmunoprecipitation experiments showing GSK3β binding to both full-length (FL) and short forms of SIRT3 in human cardiac fibroblasts (n = 3). (D) Flag-tagged [35S]SIRT3 or [35S]SIRT5 were synthesized using a TNT-Quick Coupled transcription/translation system, and their binding to GSK3β was analyzed by a GST pulldown assay. Autoradiograms in the upper panel show binding of [35S]SIRT3 to GST-GSK3β. Expression levels of SIRT3, SIRT5, and GSK3β were detected by Western blotting. Part of this figure was adapted from our previous work (input lanes 1 and 2 from Fig. 2A in reference 18). (E) Confocal microscopy showing colocalization of GSK3-β (red) and SIRT3 (green) in human cardiac fibroblasts (n = 5). (F and G) Recombinant HA-GSK3β was incubated with recombinant p300 and cofactor acetyl coenzyme A (acetyl-CoA). Following completion of the reaction, Ac-GSK3β was incubated with the recombinant SIRT3 in the presence or absence of NAD for 2 h in a deacetylation reaction buffer. GSK3β acetylation was analyzed by Western blotting (F), and enzymatic activity was determined against a glycogen synthase peptide (G). (H) GSK3β immunoprecipitated from heart lysates of non-Tg (NTg) and SIRT3-Tg mice was probed with antiacetyllysine antibody. (I) GSK3β was immunoprecipitated from heart lysates of non-Tg and Sirt3-Tg mice and analyzed for catalytic activity against the glycogen synthase peptide. Values are means ± standard error (n = 4). *, P < 0.001.
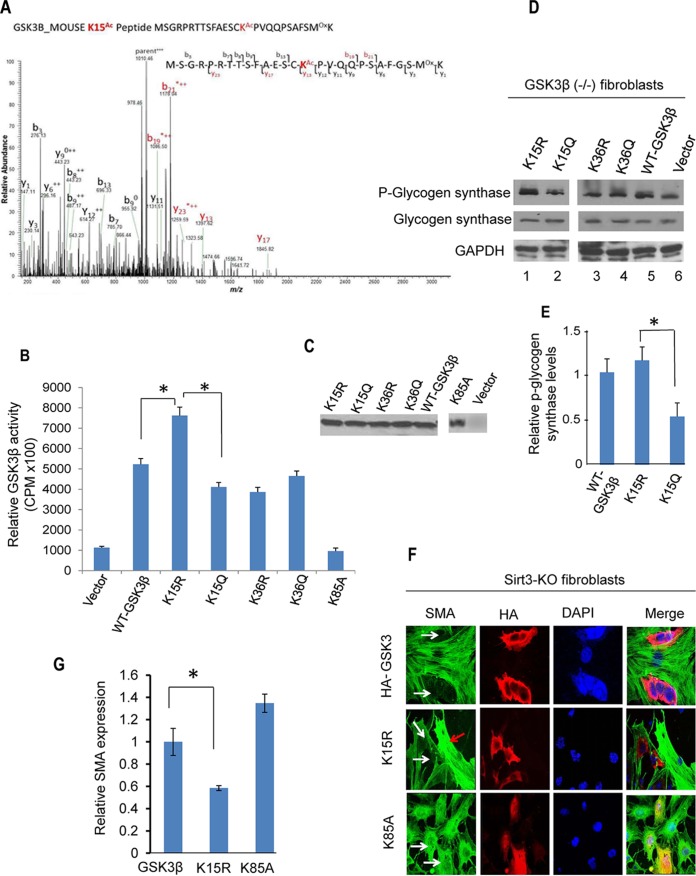
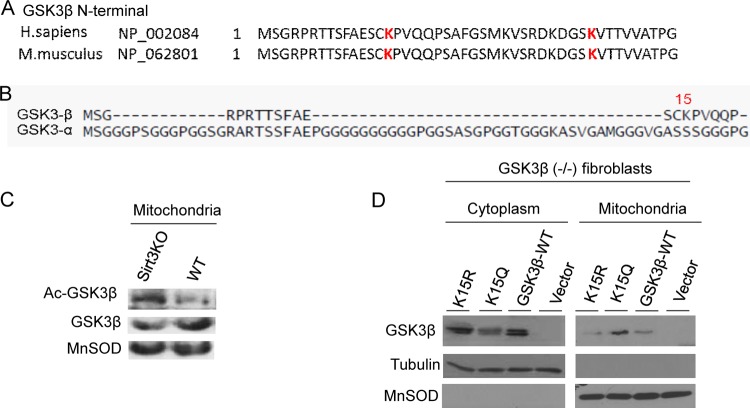
To determine how acetylation regulates GSK3β activity, we prepared GSK3β from Sirt3-KO fibroblasts and subjected it to tandem mass spectrometry (MS/MS) analysis. We identified two lysine (K) residues (K15 and K36) as acetylation sites of GSK3β (Fig. 8A) (MS/MS data of acetylated K36 [Ac-K36] not shown). Mutation of K15 to glutamine (Q; acetylation mimic) significantly reduced the enzymatic activity of GSK3β in phosphorylation of the recombinant peptide substrate whereas mutation to arginine (R; deacetylation mimic) increased this activity (Fig. 8B and C). Mutation of K36 to R did not increase activity of the kinase nor did mutation to Q decrease this activity (Fig. 8B). In this assay, a kinase-dead GSK3β-K85A mutant was used as a negative control (32). Consistent with these findings, we also observed that the GSK3β-K15R mutant had significantly higher catalytic activity than GSK3β-K15Q in phosphorylating glycogen synthase (Fig. 8D and E). These findings demonstrated that deacetylation of the K15 residue promotes GSK3β activity. To further substantiate these findings, we asked whether the deacetylation mimetic GSK3β-K15R could revert the myofibroblast phenotype of Sirt3-KO fibroblasts. Overexpression of GSK3β-K15R but not the mutant significantly reduced SMA expression in Sirt3-KO fibroblasts (Fig. 8F and G), thus demonstrating the ability of GSK3β-K15R to block transformation of Sirt3-KO fibroblasts into myofibroblasts.
FIG 8.
Lysine 15 (K15) acetylation regulates GSK3β activity. (A) Annotation of representative tandem mass spectra of trypsin-digested GSK3β, depicting K15 acetylation (in red). (B) HA-tagged wild type and K15R, K15Q, K36R, K36Q, and K85A mutants of GSK3β were purified from GSK3β null mouse embryonic fibroblasts using HA antibody-conjugated agarose beads. The enzymatic activity of GSK3β mutants was determined against a glycogen synthase peptide. Values are means ± standard errors (n = 5). *, P < 0.001. (C) Western analysis showing expression levels of different GSK3β mutants. (D) GSK3β null fibroblasts were overexpressed with different forms of GSK3β. The catalytic activity of GSK3β was assayed by measuring the phosphorylation of glycogen synthase by Western blotting. (E) Quantification of glycogen synthase phosphorylation by GSK3β-K15 mutants. Note the increased activity of GSK3β-K15R in phosphorylation of the substrate compared to that of GSK3β-K15Q. Values are means plus standard errors (n = 3). *, P < 0.01. (F) Sirt3-KO fibroblasts were overexpressed with different GSK3β constructs. Expression of SMA was determined by confocal imaging. White arrows indicate reduced SMA expression in GSK3β-WT- and GSK3β-K15R-expressing cells but not in GSK3β mutant (K85A) cells. The red arrow indicates that cells negative for GSK3β-K15R expression robustly express SMA. (G) Quantification of SMA levels in cells expressing different GSK3β mutants. Values are means ± standard errors (n = 3).
Sequence comparison analysis revealed that the K15 residue is conserved among GSK3β enzymes of different species, but it is not present in GSK3α (Fig. 9A and B). Since GSK3α has not been found in mitochondria, our findings also raised the possibility that K15 acetylation regulates GSK3β localization into mitochondria. To test this possibility, we asked first where deacetylation of GSK3β occurs in the cell. For this purpose we prepared GSK3β from mitochondria of wild-type and Sirt3-KO hearts and determined its acetylation by Western blotting. There was more acetylated GSK3β in the mitochondrial fraction of Sirt3-KO than in wild-type hearts, suggesting that deacetylation of the kinase occurs at mitochondria (Fig. 9C). To confirm these results, we overexpressed GSK3β-KO fibroblasts with different mutations of GSK3β and examined their localization in mitochondrial and cytosolic fractions of the cell. As shown in Fig. 9D, while GSK3β-K15Q (acetylation mimic) was mostly localized in mitochondria, the K15R mutant and wild-type GSK3β were present mostly in the cytosolic fraction. These results thus demonstrated that (i) acetylation of K15 promotes mitochondrial localization of GSK3β and (ii) deacetylated GSK3β is localized mostly outside mitochondria.
FIG 9.
GSK3β acetylation at residue K15 regulates its mitochondrial localization. (A) N-terminal regions of GSK3β from different vertebrate species, showing acetylated K15 and K36 residues (in red). (B) Comparison of the N-terminal regions of GSK3β and GSK3α showing that residue K15 is present only in the β-isoform. (C) GSK3β prepared from the mitochondrial fraction of the heart lysate was assayed by Western blotting using antiacetyllysine and antibodies against the indicated proteins. (D) GSK3β null mouse embryonic fibroblasts were subjected to overexpression of different versions of GSK3β. The cytoplasmic and mitochondrial fractions of cells were prepared and analyzed by Western blotting using antibodies against the indicated proteins. Blots are representative of three separate experiments. Note that K15Q is mostly present in mitochondria and that K15R is located in the cytoplasm. H. sapiens, Homo sapiens; M. musculus, Mus musculus.
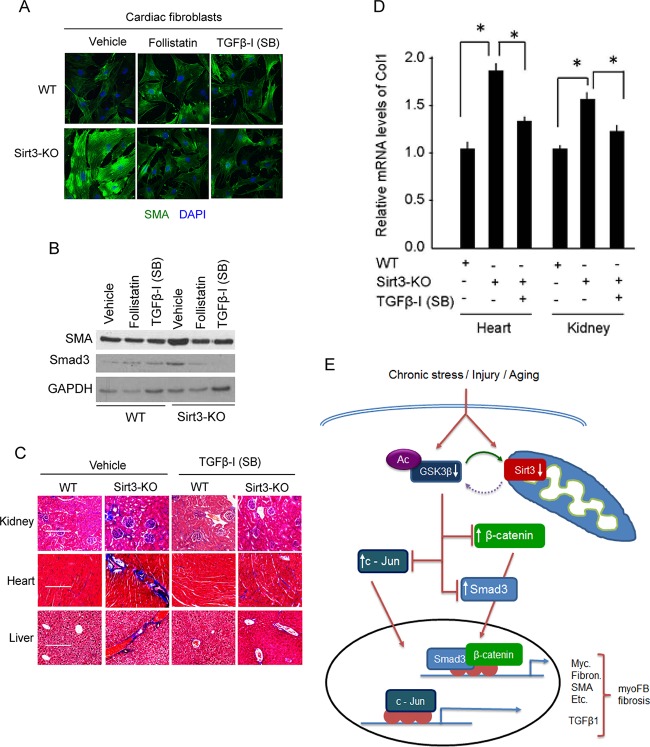
Finally, to confirm the role of TGF-β1 in the induction of fibrosis during Sirt3 deficiency, we tested the effects of TGF-β inhibitors. Sirt3-KO fibroblasts were treated with follistatin, an activin-binding protein, which neutralizes members of the TGF-β superfamily, or with a small-molecule inhibitor of TGF-β receptors, SB-505124 (SB) (33, 34). Both inhibitors significantly reduced the expression of SMA and Smad3 in Sirt3-KO fibroblasts (Fig. 10A and B). Consistent with these findings, we also found that chronic inhibition of TGF-β signaling in vivo by administration of SB-505124 (SB) reduced fibrotic changes in the kidney, liver, and heart of Sirt3-KO mice (Fig. 10C and D). These data together demonstrated that defects in regulation of TGF–GSK3–Smad3 signaling contribute to transformation of fibroblasts to myofibroblasts and induction of tissue fibrosis in Sirt3-deficient mice.
FIG 10.
TGF-β inhibitors block fibrotic changes of Sirt3-KO cells/tissues. (A) Confocal microscopy showing SMA expression in wild-type and Sirt3-KO cardiac fibroblasts treated with the TGF-β inhibitor follistatin (200 nM) or SB-505124 (1 μM) for 48 h. DAPI, 4′,6′-diamidino-2-phenylindole. (B) Western analysis showing expression levels of SMA and Smad3 in the same cells as used for the experiment described for panel A. (C) WT and Sirt3-KO mice (10 months old) were treated with vehicle or SB-505124 at a dose of 10 mg kg−1 for 2 months (three intraperitoneal injections/week). Tissue fibrosis was analyzed by Masson's trichrome staining of tissue sections (n = 7 mice per group). (D) Real-time PCR analysis of collagen 1 gene expression in different groups of mice. Values are means ± standard errors (n = 4 or 5 mice per group). *, P < 0.001. (E) A simplified scheme illustrating the role of SIRT3 in regulating GSK3β–TGFβ–Smad3 signaling and development of fibrosis. SIRT3 deacetylates GSK3β at mitochondria. In SIRT3-deficient cells GSK3β is acetylated, inhibiting its ability to phosphorylate the substrates. Decreased GSK3β-dependent phosphorylation causes stabilization of substrates like Smad3, c-Jun, and β-catenin, leading to their increased import into the nucleus, where they regulate the expression of profibrotic genes and transformation of fibroblasts into myofibroblasts.
DISCUSSION
In this study, we showed a new role for SIRT3 in retarding aging-associated tissue fibrosis. Sirt3-KO mice developed increased blood pressure and had exacerbated tissue fibrosis with age, whereas whole-body Sirt3-Tg mice showed reduced fibrosis compared to the level in WT mice. Experiments carried out to demonstrate the mechanism involved in SIRT3-mediated protection from fibrosis showed the role of GSK3β deacetylation, a new target of SIRT3. Acetylation of residue K15 reduced GSK3β activity, whereas deacetylation increased GSK3β's ability to phosphorylate its substrates. Our data also demonstrate that the antifibrotic effects of SIRT3 are independent of its ability to regulate cellular ROS levels.
Sirt3 and aging-associated fibrosis.
Aging is a degenerative process involving a multitude of factors. Though the role of sirtuins in aging has been debated, recent findings suggest that SIRT1, SIRT3, and SIRT6 have the potential to retard the aging process through independent mechanisms (35–37). SIRT3 levels have been reported to be suppressed during aging (36). Moreover, SIRT3 protein levels were found to be decreased in several disease models, where SIRT3 deficiency induces aging-associated hearing loss, propensity to develop cancer, metabolic diseases, and heart failure (9–12). Even though the susceptibilities of different tissues to fibrosis are different, they all reveal the same common characteristics at the cellular and molecular levels, which include tissue degeneration, leukocyte infiltration, inflammation, and proliferation of cells with a fibroblast-like phenotype (38). In this study, we observed that Sirt3-KO mice at around 15 months of age developed severe fibrosis in multiple organs, including liver, heart, lung, and kidney, compared to characteristics of their age-matched WT littermates. Moreover, overexpression of SIRT3 attenuated Ang-II-induced fibrotic changes both in vitro and in vivo. Whole-body Sirt3-transgenic mice showed reduced fibrosis in several tissues, including heart, liver, kidney, and lung. Consistent with this, we found that fibroblasts isolated from human diseased hearts show low SIRT3 levels, and these were associated with transformation of fibroblasts into myofibroblasts, suggesting that SIRT3 attenuates aging-associated fibrotic changes. It is worth noting that in population studies, increased activity of SIRT3 due to polymorphism was linked to human longevity, and decreased activity was associated with predisposition to develop type II diabetes, thus supporting a role of SIRT3 for retarding the human aging process (10, 39, 40).
Mitochondrial SIRT3, ROS synthesis, and fibrosis.
With regard to the mechanism involved in SIRT3-mediated cellular protection, most previous studies focused on two common mechanisms, reduced cellular ROS levels and increased bioenergetics. These mechanisms are based on the observations that SIRT3 deficiency induces defects in mitochondrial oxidative phosphorylation, leading to increased ROS production and decreased ATP synthesis from mitochondria (41). SIRT3 can also directly activate the ROS scavenger MnSOD by deacetylation, thus reducing the ROS levels (42). Additionally, it can achieve the same indirectly by deacetylating mitochondrial isocitrate dehydrogenase 2, leading to increased NADPH levels to protect cells from oxidative-stress-induced apoptosis (43). SIRT3 is also implicated in regulation of the expression of nuclear genes by controlling activity of transcription factors such as p53, PGC-1α, FoxO3a, and hypoxia-inducible factor 1α (HIF-1α) via monitoring mitochondrial ROS production (44). In this study, however, even after concerted efforts, we found no evidence to support the role of ROS generated during SIRT3 deficiency as a cause of tissue fibrosis. In our rescue experiments, constitutively active GSK3β, but not antioxidants, blocked the fibrotic changes in Sirt3-KO cells, thus excluding the role of ROS as a prime trigger of fibrosis. It is worth noting that ROS scavengers are incapable of reducing fibrosis and improving organ functions in patients (45, 46). In a similar vein, many recent reports have challenged the concept of oxidative damage as the cause of the aging process (47–49). In studies with Caenorhabditis elegans, it has been reported that while superoxide dismutase is required to survive acute stress, it is dispensable for the life span extension of the animal (47, 48). In another study, it was shown that the health-promoting effects of physical exercise for diabetic patients are not improved, but rather antagonized, by use of antioxidants (49). Similarly, antioxidant supplements in randomized clinical trials of primary and secondary preventions were shown to exert harmful effects and increase mortality (50). In light of these reports an alternative hypothesis for the antiaging effects of SIRT3 is necessary. If SIRT3 acts as an antiaging molecule, its health-promoting activity may not be limited to its ability to control mitochondrial ROS production. Rather, there must be additional mechanisms through which SIRT3 curtails aging and aging-associated diseases.
SIRT3 activates GSK3β.
We found that SIRT3 targets GSK3β to control tissue fibrosis. GSK3β is a constitutively active kinase that critically regulates diverse cellular functions involved in maintaining cellular growth, survival, and death (4). A role for GSK3β has been also implicated in controlling tissue fibrosis and the aging process (51). GSK3β is inhibited by N-terminal S9 phosphorylation by the upstream kinases. However, mutation studies have indicated that S9 mutation does not eliminate GSK3β inhibition by growth stimuli, suggesting that GSK3β is also subject to inhibition by other mechanisms (6). In this study, we found that reversible lysine acetylation regulates GSK3β activity, which is independent of its phosphorylation status. We found that GSK3β is critically acetylated at residue K15. This acetylation negatively regulates GSK3β activity to phosphorylate substrates. SIRT3 directly binds to and deacetylates GSK3β, and this modification increases the enzymatic activity of the kinase and thereby blocks the ability of factors like Smad3, c-Jun, and β-catenin to promote expression of fibrotic genes (Fig. 10E). In an earlier study acetylation of GSK3β was reported in hepatocellular carcinoma. In these cells it was shown that SIRT2, which is mostly expressed in the cytoplasm, is incapable of deacetylating the kinase (52). Another sirtuin which is implicated in regulating GSK3β activity is SIRT1. But this sirtuin analogue inhibits GSK3β activity by deacetylating and activating Akt1 in the heart (19). In our studies we found no change in GSK3β acetylation in SIRT1-KO hearts. Based on these reports and the data presented here, we believe that the deacetylation-dependent activation of GSK3β is specifically carried out by the mitochondrial deacetylase SIRT3.
Where in the cell does SIRT3 deacetylate GSK3β? GSK3β is localized in the cytoplasm, in the nucleus, and in mitochondria, where it targets compartment-specific substrates (53, 54). In the mitochondria GSK3β has been shown to target substrates present in the matrix, such as cyclophilin D and Rieske, a subunit of complex III, and those localized at the level of two membranes, such as hexokinase II (HK II), myeloid cell leukemia 2 (Mcl-2), Bcl-2, Bax, Noxa, voltage-dependent anion channel (VDAC), and adenine nucleotide transporter (ANT) (5). The mitochondrial localization signal of GSK3β has not been defined yet. But in a recent study a role of residue K15 in the transport of GSK3β into mitochondria has been suggested (55). Consistent with our findings, mutation of K15 to alanine was shown to suppress mitochondrial localization of GSK3β in H9C2 cells (55). SIRT3 is a mitochondrial protein, and within mitochondria it is present in the matrix as well as in the inner membrane space, where it targets OPA1 to regulate mitochondrial dynamics (18, 56). In this study, we found that transgenic mice expressing mitochondrial SIRT3 were capable of blocking fibrosis, together with decreasing GSK3β acetylation and reducing TGFβ1 and Smad3 levels. Moreover, the deacetylated form of GSK3β was localized mostly outside mitochondria. Exactly where GSK3β is deacetylated in mitochondria must await characterization of the mechanisms regulating GSK3β transport in and out of mitochondria. Based on our findings and reports from others, we believe that GSK3β is deacetylated at the mitochondria and transported to the cytosol to regulate its substrates. This could be similar to mechanisms involved in the activation of another SIRT3 deacetylation target, Skp2, which is exported from the mitochondria after deacetylation to regulate nuclear gene expression (57).
SIRT3 and TGF-β1 signaling.
A role of GSK3β in regulating TGF-β1 synthesis has been previously documented (31). TGF-β is one of the cytokines released during the inflammatory response which activates fibroblasts to transform into myofibroblasts to produce ECM. Excessive production of TGF-β1 was linked to tissue fibrosis during aging and pathological changes in many organs, including liver, lung, kidney, heart, skeletal muscle, and uterus (58–60). However, the molecular basis of this increased synthesis of TGF-β was unknown. In the present study, we found that SIRT3 can negatively regulate TGF-β synthesis at the promoter level. Sirt3-deficient cells exhibited increased expression of TGF-β1 mRNA and promoter activity, whereas overexpression of SIRT3 blocked the activation of this promoter. Cell signaling analysis revealed that this was achieved indirectly by negatively regulating expression of the nuclear transcription factor (like c-Jun) but not by directly suppressing the gene promoter since CHIP analysis showed no binding of SIRT3 to the promoters of TGF-β1. Thus, our data elucidate the molecular link, especially the link between the mitochondria and the nucleus, involved in excess TGF-β1 production during aging-associated tissue fibrosis.
Growing evidence in the field of longevity suggests that dietary restriction retards the aging process and activates SIRT3 while antagonizing TGF-β signaling (8, 22). Our findings suggest that both of these pathways are directly linked at the molecular level. Previous studies have demonstrated that in addition to SIRT3, SIRT1 and SIRT6 are also activated in response to dietary restriction (7). SIRT1 has been shown to exert tissue-specific effects with regard to its ability to regulate tissue fibrosis. SIRT1 inhibits TGF-β-mediated renal fibrosis and blocks the epithelial-to-mesenchymal transition that leads to organ fibrosis (61, 62). In contrast to this, other studies report that SIRT1-deficient hearts develop reduced fibrosis following stress, while SIRT1-overexpressing hearts develop massive fibrosis with age (19, 63). Another sirtuin, SIRT6, antagonizes TGF-β-induced senescence in human bronchial epithelial cells (64). However, whether SIRT6 plays a role in regulation of endogenous TGF-β1 expression is currently unknown. Based on the findings of the present work, we believe that SIRT3 can indirectly block TGF-β expression by regulating acetylation of GSK3β and thereby controlling the aging and aging-associated fibrotic remodeling of the tissue.
ACKNOWLEDGMENTS
We thank F. W. Alt (Harvard Medical School, Boston, MA) for providing Sirt3-KO mice. GSK3β-KO fibroblasts were kindly provided by James Woodgett, Mount Sinai Hospital, Toronto, Canada. We also thank J. Shin, Department of Pediatrics, for her help in doing ChIP assays, Karen DeSouza, Department of Surgery, for helping us in analyzing human cardiac fibroblasts, and N. Dulin, Department of Medicine, for providing the TARE-Luc reporter plasmid.
This study was supported by U.S. National Institutes of Health grants RO1 HL117041 and HL111455 to M.P.G.
N.R.S. and S.B. performed the majority of the experiments. M.G. performed real-time PCR analysis and assessed catalytic activity of GSK3β mutants. S.S. performed in vitro protein binding assays. V.B.P. performed flow cytometry and TGF-β1 inhibitor experiments and participated in writing of the manuscript. R.S.N. analyzed the GSK3β signaling in cardiac lysates. D.W. did MS/MS analysis. Y.P., J.-Y.H., and E.V. generated whole-body SIRT3-transgenic mice and provided tissue samples for fibrotic analysis. M.P.G. coordinated with different investigators, supervised the whole study, and generated the final draft of the manuscript.
REFERENCES
- 1.Mehal WZ, Iredale J, Friedman SL. 2011. Scraping fibrosis: expressway to the core of fibrosis. Nat Med 17:552–553. doi: 10.1038/nm0511-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wynn TA, Ramalingam TR. 2012. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krenning G, Zeisberg EM, Kalluri R. 2010. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol 225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frame S, Cohen P. 2001. GSK3 takes centre stage more than 20 years after its discovery. Biochem J 359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiara F, Rasola A. 2013. GSK-3 and mitochondria in cancer cells. Front Oncol 3:16. doi: 10.3389/fonc.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuda T, Zhai P, Maejima Y, Hong C, Gao S, Tian B, Goto K, Takagi H, Tamamori-Adachi M, Kitajima S, Sadoshima J. 2008. Distinct roles of GSK-3α and GSK-3β phosphorylation in the heart under pressure overload. Proc Natl Acad Sci U S A 105:20900–20905. doi: 10.1073/pnas.0808315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarente L. 2011. Franklin H. Epstein lecture: sirtuins, aging, and medicine. N Engl J Med 364:2235–2244. doi: 10.1056/NEJMra1100831. [DOI] [PubMed] [Google Scholar]
- 8.Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, Westphall MS, Pagliarini DJ, Prolla TA, Assadi-Porter F, Roy S, Denu JM, Coon JJ. 2013. Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell 49:186–199. doi: 10.1016/j.molcel.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finley LW, Carracedo A, Lee J, Souza A, Egia A, Zhang J, Teruya-Feldstein J, Moreira PI, Cardoso SM, Clish CB, Pandolfi PP, Haigis MC. 2011. SIRT3 opposes reprogramming of cancer cell metabolism through HIF1α destabilization. Cancer Cell 19:416–428. doi: 10.1016/j.ccr.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B, Stevens RD, Muehlbauer MJ, Kakar S, Bass NM, Kuusisto J, Laakso M, Alt FW, Newgard CB, Farese RV Jr, Kahn CR, Verdin E. 2011. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell 44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. 2010. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell 143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. 2009. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown K, Xie S, Qiu XL, Mohrin M, Shin JY, Liu YF, Zhang D, Scadden DT, Chen D. 2013. SIRT3 reverses aging-associated degeneration. Cell Rep 3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwer B, North BJ, Frye RA, Ott M, Verdin E. 2002. The human silent information regulator (Sir) 2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol 158:647–657. doi: 10.1083/jcb.200205057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D'Souza KM, Malhotra R, Philip JL, Staron ML, Theccanat T, Jeevanandam V, Akhter SA. 2011. G protein-coupled receptor kinase-2 is a novel regulator of collagen synthesis in adult human cardiac fibroblasts. J Biol Chem 286:15507–15516. doi: 10.1074/jbc.M111.218263. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Sundaresan NR, Vasudevan P, Zhong L, Kim G, Samant S, Parekh V, Pillai VB, Ravindra PV, Gupta M, Jeevanandam V, Cunningham JM, Deng CX, Lombard DB, Mostoslavsky R, Gupta MP. 2012. The sirtuin SIRT6 blocks IGF-Akt signaling and development of cardiac hypertrophy by targeting c-Jun. Nat Med 18:1643–1650. doi: 10.1038/nm.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown KD, Maqsood S, Huang JY, Pan Y, Harkcom W, Li W, Sauve A, Verdin E, Jaffrey SR. 2014. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab 20:1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samant SA, Zhang HJ, Hong Z, Pillai VB, Sundaresan NR, Wolfgeher D, Archer SL, Chan DC, Gupta MP. 2014. SIRT3 deacetylates and activates OPA1 to regulate mitochondrial dynamics during stress. Mol Cell Biol 34:807–819. doi: 10.1128/MCB.01483-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundaresan NR, Pillai VB, Wolfgeher D, Samant S, Vasudevan P, Parekh V, Raghuraman H, Cunningham JM, Gupta M, Gupta MP. 2011. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal 4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 20.Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP. 2008. SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 28:6384–6401. doi: 10.1128/MCB.00426-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS. 2010. TGFβ signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7:62. doi: 10.1186/1742-2094-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meyer TE, Kovacs SJ, Ehsani AA, Klein S, Holloszy JO, Fontana L. 2006. Long-term caloric restriction ameliorates the decline in diastolic function in humans. J Am Coll Cardiol 47:398–402. doi: 10.1016/j.jacc.2005.08.069. [DOI] [PubMed] [Google Scholar]
- 23.Cobbs SL, Gooch JL. 2007. NFATc is required for TGFP-mediated transcriptional regulation of fibronectin. Biochem Biophys Res Commun 362:288–294. doi: 10.1016/j.bbrc.2007.07.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. 2009. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol 20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ichikawa T, Sugiura H, Koarai A, Kikuchi T, Hiramatsu M, Kawabata H, Akamatsu K, Hirano T, Nakanishi M, Matsunaga K, Minakata Y, Ichinose M. 2013. 25-hydroxycholesterol promotes fibroblast-mediated tissue remodeling through NF-κB dependent pathway. Exp Cell Res 319:1176–1186. doi: 10.1016/j.yexcr.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Tipton DA, Woodard ES, Baber MA, Dabbous MK. 2004. Role of the c-myc proto-oncogene in the proliferation of hereditary gingival fibromatosis fibroblasts. J Periodontol 75:360–369. doi: 10.1902/jop.2004.75.3.360. [DOI] [PubMed] [Google Scholar]
- 27.Iwahara T, Bonasio R, Narendra V, Reinberg D. 2012. SIRT3 functions in the nucleus in the control of stress-related gene expression. Mol Cell Biol 32:5022–5034. doi: 10.1128/MCB.00822-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rong Y, Doctrow SR, Tocco G, Baudry M. 1999. EUK-134, a synthetic superoxide dismutase and catalase mimetic, prevents oxidative stress and attenuates kainate-induced neuropathology. Proc Natl Acad Sci U S A 96:9897–9902. doi: 10.1073/pnas.96.17.9897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zafarullah M, Li WQ, Sylvester J, Ahmad M. 2003. Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 60:6–20. doi: 10.1007/s000180300001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue Y, Itoh Y, Abe K, Okamoto T, Daitoku H, Fukamizu A, Onozaki K, Hayashi H. 2007. Smad3 is acetylated by p300/CBP to regulate its transactivation activity. Oncogene 26:500–508. doi: 10.1038/sj.onc.1209826. [DOI] [PubMed] [Google Scholar]
- 31.Guo X, Ramirez A, Waddell DS, Li ZZ, Liu XD, Wang XF. 2008. Axin and GSK3-beta control Smad3 protein stability and modulate TGF-beta signaling. Gene Dev 22:106–120. doi: 10.1101/gad.1590908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora-Santos M, Limon-Mortes MC, Giraldez S, Herrero-Ruiz J, Saez C, Japon MA, Tortolero M, Romero F. 2011. Glycogen synthase kinase-3β (GSK3β) negatively regulates PTTG1/human securin protein stability, and GSK3β inactivation correlates with securin accumulation in breast tumors. J Biol Chem 286:30047–30056. doi: 10.1074/jbc.M111.232330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DaCosta Byfield S, Major C, Laping NJ, Roberts AB. 2004. SB-505124 is a selective inhibitor of transforming growth factor-beta type I receptors ALK4, ALK5, and ALK7. Mol Pharmacol 65:744–752. doi: 10.1124/mol.65.3.744. [DOI] [PubMed] [Google Scholar]
- 34.Massague J, Chen YG. 2000. Controlling TGF-beta signaling. Genes Dev 14:627–644. [PubMed] [Google Scholar]
- 35.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. 2012. The sirtuin SIRT6 regulates lifespan in male mice. Nature 483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- 36.Lanza IR, Short DK, Short KR, Raghavakaimal S, Basu R, Joyner MJ, McConnell JP, Nair KS. 2008. Endurance exercise as a countermeasure for aging. Diabetes 57:2933–2942. doi: 10.2337/db08-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. 2013. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab 18:416–430. doi: 10.1016/j.cmet.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wynn TA. 2008. Cellular and molecular mechanisms of fibrosis. J Pathol 214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, Franceschi C, Passarino G, De Benedictis G. 2005. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics 85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, Passarino G, Feraco E, Mari V, Barbi C, BonaFe M, Franceschi C, Tan Q, Boiko S, Yashin AI, De Benedictis G. 2003. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol 38:1065–1070. doi: 10.1016/S0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- 41.Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. 2008. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A 105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tao R, Vassilopoulos A, Parisiadou L, Yan Y, Gius D. 2014. Regulation of MnSOD enzymatic activity by Sirt3 connects the mitochondrial acetylome signaling networks to aging and carcinogenesis. Antioxid Redox Signal 20:1646–1654. doi: 10.1089/ars.2013.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu W, Dittenhafer-Reed KE, Denu JM. 2012. SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem 287:14078–14086. doi: 10.1074/jbc.M112.355206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sack MN, Finkel T. 2012. Mitochondrial metabolism, sirtuins, and aging. Cold Spring Harb Perspect Biol 4:a013102. doi: 10.1101/cshperspect.a013102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Griese M, Kappler M, Eismann C, Ballmann M, Junge S, Rietschel E, van Koningsbruggen-Rietschel S, Staab D, Rolinck-Werninghaus C, Mellies U, Kohnlein T, Wagner T, Konig S, Teschler H, Heuer HE, Kopp M, Heyder S, Hammermann J, Kuster P, Honer M, Mansmann U, Beck-Speier I, Hartl D, Fuchs C, Hector A, Grp GS. 2013. Inhalation treatment with glutathione in patients with cystic fibrosis a randomized clinical trial. Am J Respir Crit Care 188:83–89. doi: 10.1164/rccm.201303-0427OC. [DOI] [PubMed] [Google Scholar]
- 46.Idiopathic Pulmonary Fibrosis Clinical Research Network Raghu G, Anstrom KJ, King TE, Lasky JA, Martinez FJ. 2012. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med 366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabreiro F, Ackerman D, Doonan R, Araiz C, Back P, Papp D, Braeckman BP, Gems D. 2011. Increased life span from overexpression of superoxide dismutase in Caenorhabditis elegans is not caused by decreased oxidative damage. Free Radic Biol Med 51:1575–1582. doi: 10.1016/j.freeradbiomed.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doonan R, McElwee JJ, Matthijssens F, Walker GA, Houthoofd K, Back P, Matscheski A, Vanfleteren JR, Gems D. 2008. Against the oxidative damage theory of aging: superoxide dismutases protect against oxidative stress but have little or no effect on life span in Caenorhabditis elegans. Genes Dev 22:3236–3241. doi: 10.1101/gad.504808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. 2009. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. 2007. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 51.Jin J, Wang GL, Timchenko L, Timchenko NA. 2009. GSK3β and aging liver. Aging 1:582–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen J, Chan AW, To KF, Chen W, Zhang Z, Ren J, Song C, Cheung YS, Lai PB, Cheng SH, Ng MH, Huang A, Ko BC. 2013. SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3β/β-catenin signaling. Hepatology 57:2287–2298. doi: 10.1002/hep.26278. [DOI] [PubMed] [Google Scholar]
- 53.Hoshi M, Sato M, Kondo S, Takashima A, Noguchi K, Takahashi M, Ishiguro K, Imahori K. 1995. Different localization of tau protein kinase I/glycogen synthase kinase-3β from glycogen synthase kinase-3α in cerebellum mitochondria. J Biochem 118:683–685. [DOI] [PubMed] [Google Scholar]
- 54.Petit-Paitel A, Brau F, Cazareth J, Chabry J. 2009. Involvement of cytosolic and mitochondrial GSK-3β in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS One 4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanno M, Kuno A, Ishikawa S, Miki T, Kouzu H, Yano T, Murase H, Tobisawa T, Ogasawara M, Horio Y, Miura T. 2014. Translocation of glycogen synthase kinase-3β (GSK-3β), a trigger of permeability transition, is kinase activity-dependent and mediated by interaction with voltage-dependent anion channel 2 (VDAC2). J Biol Chem 289:29285–29296. doi: 10.1074/jbc.M114.563924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi T, Wang F, Stieren E, Tong Q. 2005. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem 280:13560–13567. doi: 10.1074/jbc.M414670200. [DOI] [PubMed] [Google Scholar]
- 57.Inuzuka H, Gao D, Finley LW, Yang W, Wan L, Fukushima H, Chin YR, Zhai B, Shaik S, Lau AW, Wang Z, Gygi SP, Nakayama K, Teruya-Feldstein J, Toker A, Haigis MC, Pandolfi PP, Wei W. 2012. Acetylation-dependent regulation of Skp2 function. Cell 150:179–193. doi: 10.1016/j.cell.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalluri R, Neilson EG. 2003. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 112:1776–1784. doi: 10.1172/JCI200320530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leask A, Abraham DJ. 2004. TGF-β signaling and the fibrotic response. FASEB J 18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 60.Samarakoon R, Overstreet JM, Higgins PJ. 2013. TGF-beta signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities. Cell Signal 25:264–268. doi: 10.1016/j.cellsig.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang XZ, Wen D, Zhang M, Xie Q, Ma L, Guan Y, Ren Y, Chen J, Hao CM. 2014. Sirt1 activation ameliorates renal fibrosis by inhibiting the TGF-β/Smad3 pathway. J Cell Biochem 115:996–1005. doi: 10.1002/jcb.24748. [DOI] [PubMed] [Google Scholar]
- 62.Simic P, Williams EO, Bell EL, Gong JJ, Bonkowski M, Guarente L. 2013. SIRT1 suppresses the epithelial-to-mesenchymal transition in cancer metastasis and organ fibrosis. Cell Rep 3:1175–1186. doi: 10.1016/j.celrep.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oka S, Zhai P, Alcendor R, Park JY, Tian B, Sadoshima J. 2012. Suppression of ERR targets by a PPARα/Sirt1 complex in the failing heart. Cell Cycle 11:856–864. doi: 10.4161/cc.11.5.19210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Minagawa S, Araya J, Numata T, Nojiri S, Hara H, Yumino Y, Kawaishi M, Odaka M, Morikawa T, Nishimura SL, Nakayama K, Kuwano K. 2011. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol 300:L391–L401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]