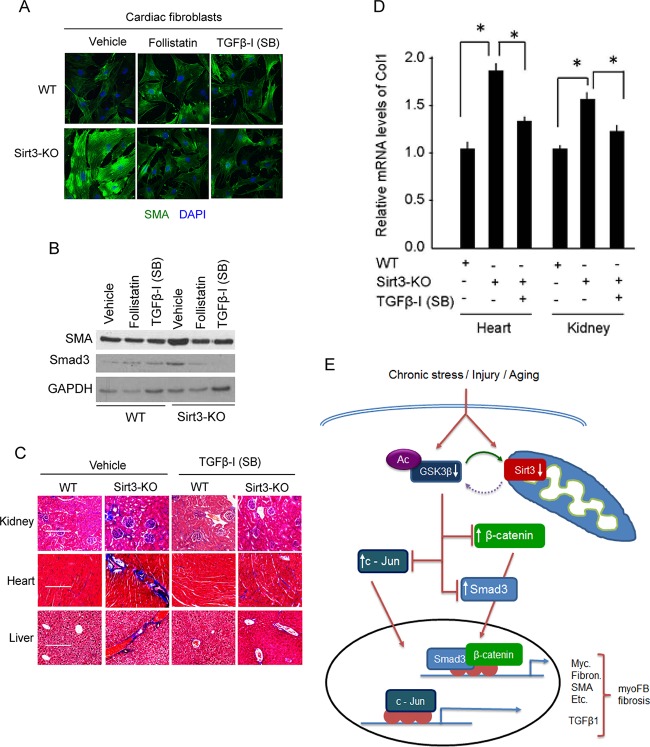
FIG 10.
TGF-β inhibitors block fibrotic changes of Sirt3-KO cells/tissues. (A) Confocal microscopy showing SMA expression in wild-type and Sirt3-KO cardiac fibroblasts treated with the TGF-β inhibitor follistatin (200 nM) or SB-505124 (1 μM) for 48 h. DAPI, 4′,6′-diamidino-2-phenylindole. (B) Western analysis showing expression levels of SMA and Smad3 in the same cells as used for the experiment described for panel A. (C) WT and Sirt3-KO mice (10 months old) were treated with vehicle or SB-505124 at a dose of 10 mg kg−1 for 2 months (three intraperitoneal injections/week). Tissue fibrosis was analyzed by Masson's trichrome staining of tissue sections (n = 7 mice per group). (D) Real-time PCR analysis of collagen 1 gene expression in different groups of mice. Values are means ± standard errors (n = 4 or 5 mice per group). *, P < 0.001. (E) A simplified scheme illustrating the role of SIRT3 in regulating GSK3β–TGFβ–Smad3 signaling and development of fibrosis. SIRT3 deacetylates GSK3β at mitochondria. In SIRT3-deficient cells GSK3β is acetylated, inhibiting its ability to phosphorylate the substrates. Decreased GSK3β-dependent phosphorylation causes stabilization of substrates like Smad3, c-Jun, and β-catenin, leading to their increased import into the nucleus, where they regulate the expression of profibrotic genes and transformation of fibroblasts into myofibroblasts.