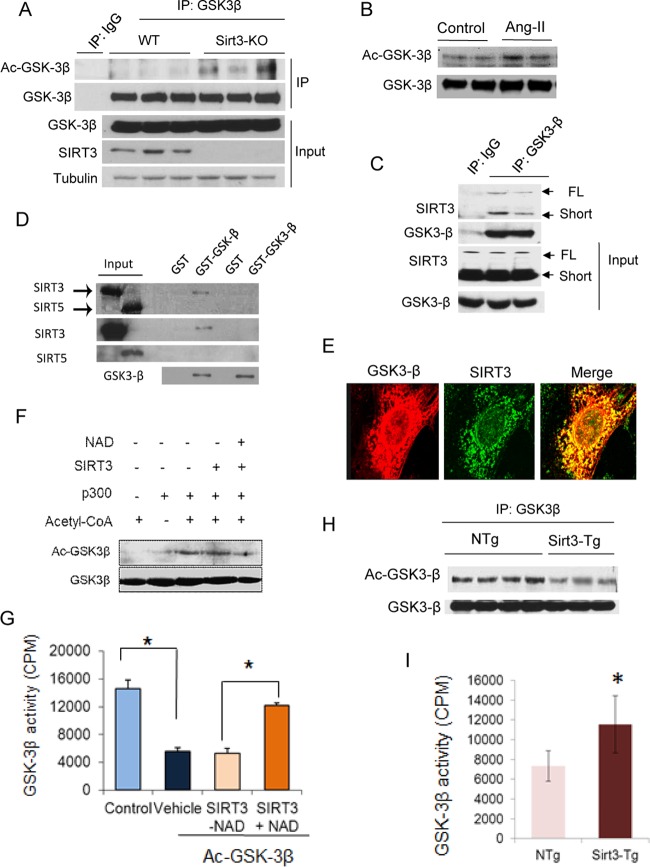
FIG 7.
SIRT3 deacetylates and activates GSK3β. (A and B) GSK3β was immunoprecipitated from heart lysates of WT and Sirt3-KO mice (A) or mice stimulated to develop hypertrophy by Ang-II infusion (B). The precipitate was analyzed by Western blot analysis using antiacetyllysine antibody. (C) Coimmunoprecipitation experiments showing GSK3β binding to both full-length (FL) and short forms of SIRT3 in human cardiac fibroblasts (n = 3). (D) Flag-tagged [35S]SIRT3 or [35S]SIRT5 were synthesized using a TNT-Quick Coupled transcription/translation system, and their binding to GSK3β was analyzed by a GST pulldown assay. Autoradiograms in the upper panel show binding of [35S]SIRT3 to GST-GSK3β. Expression levels of SIRT3, SIRT5, and GSK3β were detected by Western blotting. Part of this figure was adapted from our previous work (input lanes 1 and 2 from Fig. 2A in reference 18). (E) Confocal microscopy showing colocalization of GSK3-β (red) and SIRT3 (green) in human cardiac fibroblasts (n = 5). (F and G) Recombinant HA-GSK3β was incubated with recombinant p300 and cofactor acetyl coenzyme A (acetyl-CoA). Following completion of the reaction, Ac-GSK3β was incubated with the recombinant SIRT3 in the presence or absence of NAD for 2 h in a deacetylation reaction buffer. GSK3β acetylation was analyzed by Western blotting (F), and enzymatic activity was determined against a glycogen synthase peptide (G). (H) GSK3β immunoprecipitated from heart lysates of non-Tg (NTg) and SIRT3-Tg mice was probed with antiacetyllysine antibody. (I) GSK3β was immunoprecipitated from heart lysates of non-Tg and Sirt3-Tg mice and analyzed for catalytic activity against the glycogen synthase peptide. Values are means ± standard error (n = 4). *, P < 0.001.