Figure 4.
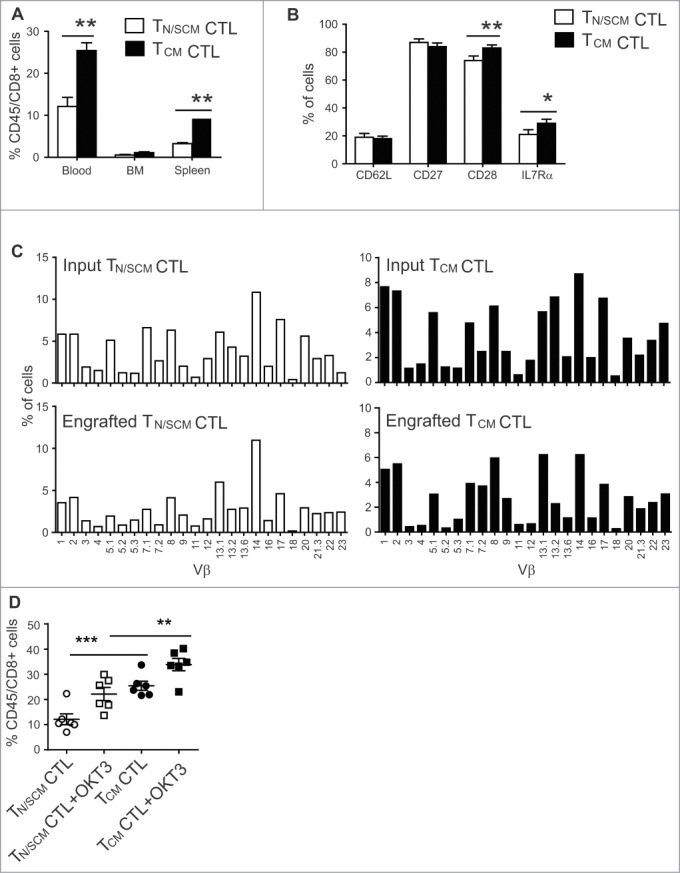
Effector cells derived from CD8+TCM precursors exhibited superior engraftment fitness in NSG mice. After 14 d of in vitro stimulation, effector cells derived from CD8+ TN/SCM (TN/SCM CTL) and CD8+TCM (TCM CTL) (107) were infused i.v. into huIL-15-replete NSG mice. (A) Three weeks after adoptive transfer, human T cells in the peripheral blood, BM and spleen of recipient mice were determined by flow cytometric analysis using antibodies specific for human CD45 and CD8. Means+SEM of a total of 6 mice per T cell subset in a representative experiment are depicted. **p < 0.01, using a Mann–Whitney test. (B) Percentages of CD62L, CD27, CD28, and IL7Rα positive cells on gated human CD45+ positive cells of pooled peripheral blood, BM and spleens are indicated. *p < 0.05 **p < 0.01 using a Mann–Whitney test. (C) TCR vβ repertoire of the effectors derived from CD8+ TN/SCM and CD8+TCM before and after infusion. Percentage (%) of human CD3+ cells that were positive for the indicated TCR vβ genes was determined by flow cytometry. (D) For in vivo stimulation, 107 irradiated OKT3-expressing LCL (+OKT3) were injected i.v. into mice that had been engrafted (3 days) with CD8+ TN/SCM or CD8+TCM derived CTLs. Human T cells in peripheral blood were determined 7 d post in vivo challenge. ***p < 0.001. **p < 0.01.
