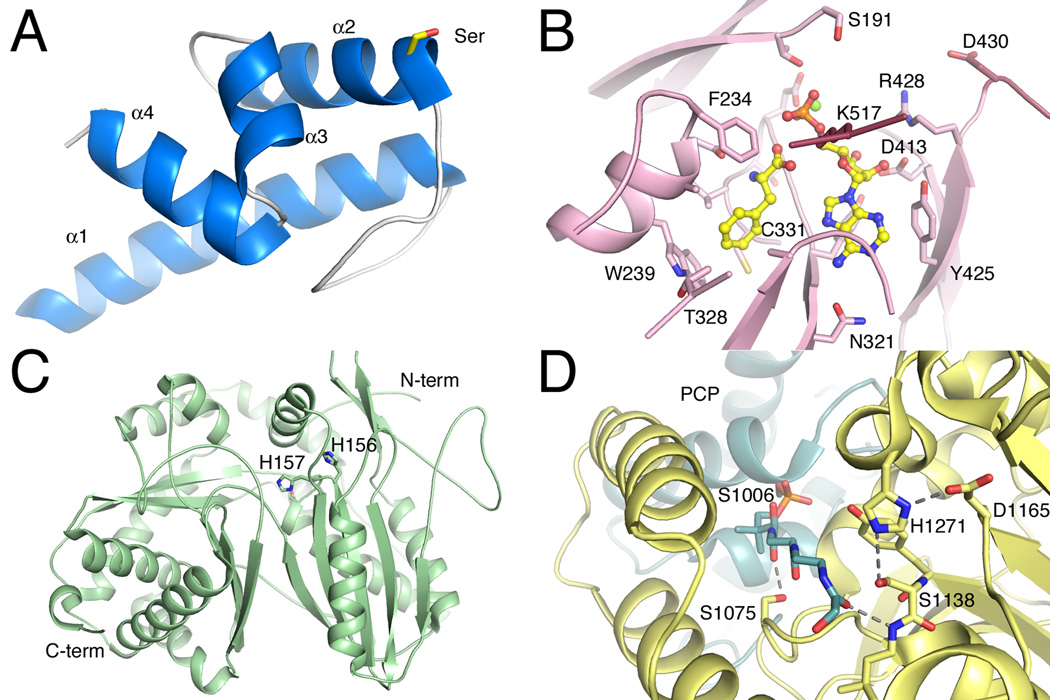
Figure 2.
Structures of core NRPS domains. A. The structure of the Type II PCP domain BlmI (PDB 4I4D). The four helices are shown along with Ser44, the site of phosphopantetheinylation. B. The active site of PheA (PDB 1AMU), the adenylation domain from the gramicidin synthetase NRPS. The ligand molecules AMP and phenylalanine are shown in ball-and-stick represenatation. Protein side chains are labeled including the residues that form the phenylalanine binding pocket and residues that interact with the nucleotide. C. The condensation domain of the CDA synthetase (PDB 4JN3) is shown in ribbon representation. The two subdomains are shown, along with active site residues His156, His157, and Asp161, which is partially obscured by His157. D. The active site of the thioesterase domain from EntF is shown (PDB 3TEJ). The pantetheine, covalently bound to Ser1006, is directed from the PCP domain to the active site, which is composed of the catalytic triad Asp1165, His1271, and Ser1138. The different NRPS domains are shown in specific colors, which will be maintained through the chapter. PCP domains are shown in blue. Adenylation domains are shown in pink for the N-terminal sub-domain and maroon for the C-terminal sub-domain. Condensation domain is shown in light green and the thioesterase domain is shown in yellow.