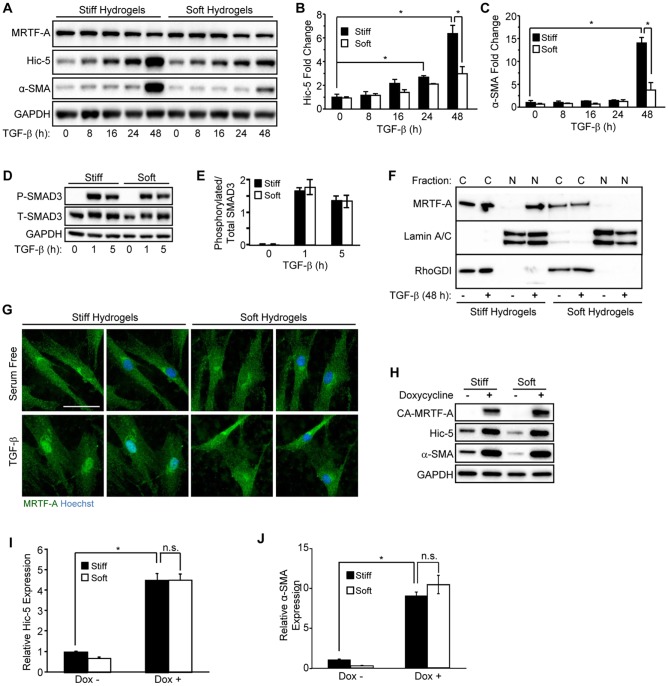
Fig. 4.
Extracellular stiffness regulates induction of Hic-5 and α-SMA through nuclear accumulation of MRTF-A in response to TGF-β. (A–C) NHDFs were cultured on stiff or soft PAHGs with 10 ng/ml of TGF-β for the indicated time points, lysed and analyzed by western blotting (representative blot, n=3). (B,C) Pooled data are shown for (B) Hic-5 and (C) α-SMA. (D) NHDFs cultured on stiff or soft PAHGs and incubated (0, 1 and 5 h) with TGF-β were lysed and subjected to western blotting for phosphorylated SMAD3 (P-SMAD3) and total SMAD3 (T-SMAD3). (E) Pooled data (n=3) are shown expressing the relative phosphorylation of SMAD3 as a ratio of the phosphorylated to total SMAD3 levels. (F) NHDFs that had been cultured (48 h) on soft or stiff PAHGs (with or without TGF-β) were extracted (see Materials and Methods section) and analyzed by western blotting for nuclear (‘N’) and cytoplasmic (‘C’) MRTF-A. Lamin A/C and RhoGDI and were used as nuclear and cytoplasmic loading controls, respectively. (G) NHDFs that had been treated as described in panel F were immunostained for MRTF-A (green) and co-stained with Hoechst 33342 (blue). Scale bar: 50 µm. (H) NHDFs that had been infected with a lentivirus encoding a doxycycline-inducible constitutively active MRTF-A construct (CA-MRTF-A) were cultured on soft or stiff PAHGs in serum-free medium with or without doxycycline (0.5 µg/ml) for 24 h. (I,J) Pooled data taken from the blot in H for (I) Hic-5 and (J) α-SMA (n=3). Statistical significance was determined by two-way ANOVA followed by Tukey's post-hoc analysis (*P<0.05) for panels B,C,E,I,J. Error bars are ±s.e.m. n.s., not significant.