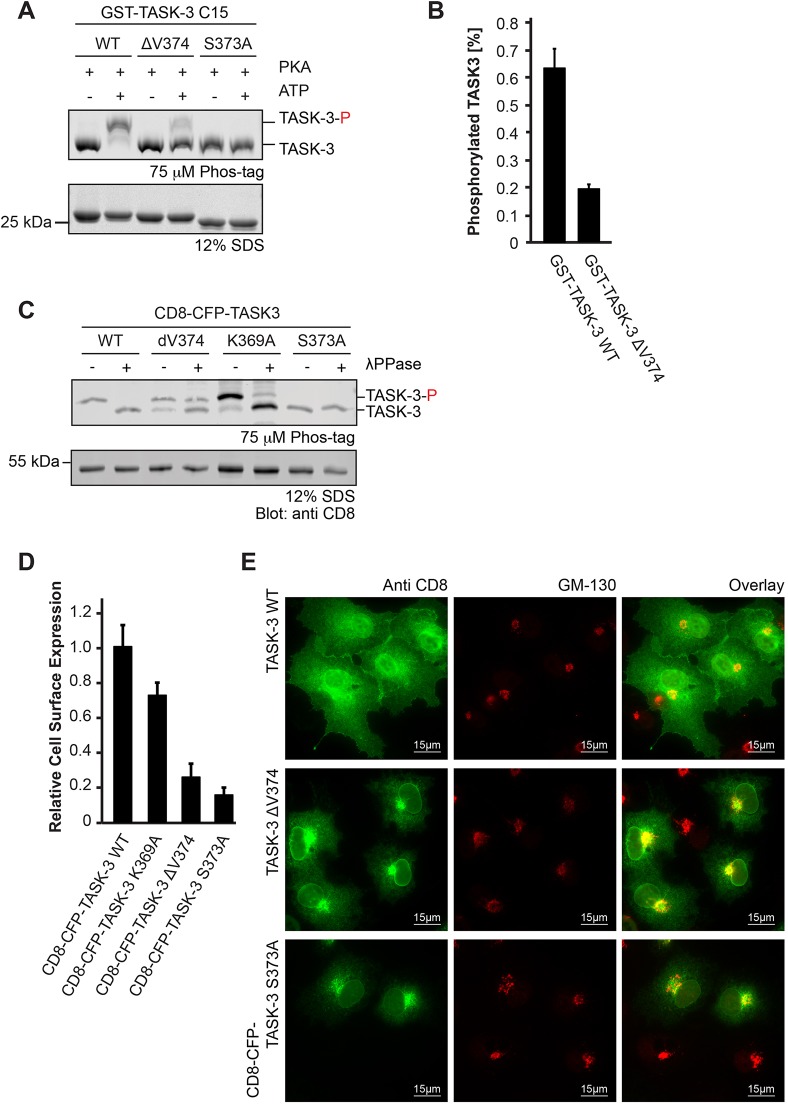
Fig. 4.
Reduced 14-3-3 binding affinity does not explain the lack of cell surface transport of the TASK-3 ΔV mutant. (A) In vitro phosphorylation of recombinant GST–TASK-3 (15 C-terminal residues) wild-type (WT), GST–TASK-3 ΔV374 and GST–TASK-3 S373A fusion proteins with the PKA catalytic subunit. Samples were resolved on an SDS-PAGE gel containing 75 µM Phostag reagent and 75 µM MnCl2, which retards the migration of proteins by chelating phosphorylated amino acid side chains. The Coomassie-stained gel is representative of six independent experiments. TASK-3-P, phosphorylated TASK-3. (B) Bar diagram of the relative amount of phosphorylated TASK-3 trafficking control region constructs shown in A. n=6, error bars depict s.e.m. (C) In vivo phosphorylation status of the indicated CD8–CFP reporter proteins, as reflected by migration in Phostag SDS-PAGE gels. λPPase, λ phosphatase. The blot is representative of 11 independent transfections. (D) Flow cytometry assay to determine the effect of the indicated TASK C-termini on the cell surface expression of a CD8–CFP reporter protein. Cells were transfected with the same series of constructs in three independent experiments. For each experiment, 10,000 cells per construct were analyzed. The mean of the CD8 signal was expressed as a ratio with the mean of the CFP signal in CFP-positive cells and normalized to that of the wild-type TASK-3 construct. Error bars depict s.e.m. (E) Subcellular localization of CD8–CFP–TASK-3 ΔV374 is indistinguishable from that of CD8–CFP–TASK-3 S373A. Constructs were transfected in three independent experiments and ca. 100 cells per transfection were analyzed by indirect immunostaining against the indicated antigens.