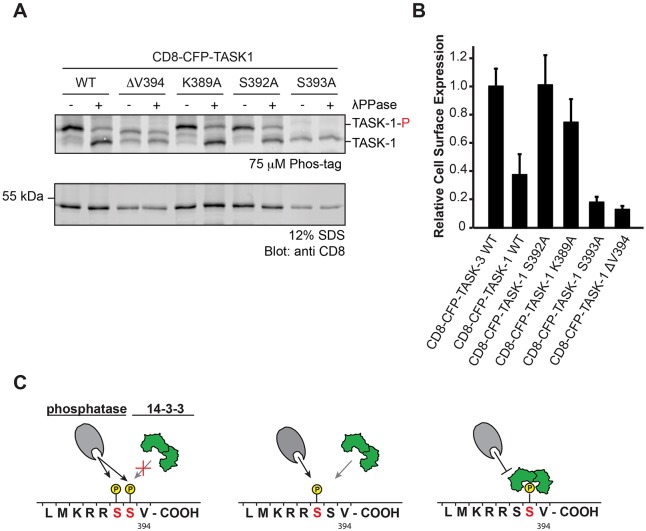
Fig. 6.
Phosphorylation of S392 in TASK-1 reduces cell surface expression. (A) In vivo phosphorylation status of the CD8 reporter protein as reflected by migration in Phostag SDS-PAGE gels. The blot is representative of seven independent transfections. TASK-1-P, phosphorylated TASK-1. (B) Flow cytometry assay to determine the effect of replacing either S392 or S393 in the TASK-1 C-terminus on the cell surface expression of the indicated CD8–CFP reporter proteins. Cells were transfected with the same series of constructs in three independent experiments. For each experiment, 10,000 cells per construct were analyzed. The mean of the CD8 signal is expressed as a ratio with the mean of the CFP signal in CFP-positive cells and normalized to that for the wild-type TASK-3 construct. Error bars depict s.e.m. (C) Model of the effect of phosphorylation of S392 or S393, or both on 14-3-3 binding and on the access of phosphatases. Note that only the population of TASK-1 reporter proteins phosphorylated on only S393 is expected to be protected from phosphatase action.